

Site Search
Search within product
第755号 2023(R05).11発行
Click here for PDF version
§Jコート肥料を用いた水稲の全量基肥施肥と被覆樹脂殻の崩壊性
Nagano Prefectural Agricultural Experiment Station
上原 敬義
吉川 直人
§土のはなし-第26回農業と環境問題-その1
わが国の窒素循環の問題点
前 ジェイカムアグリ株式会社
北海道支店 技術顧問
松中 照夫
Paddy rice with J-coat fertilizer
全量基肥施肥と被覆樹脂殻の崩壊性
Nagano Prefectural Agricultural Experiment Station
上原 敬義
吉川 直人
Introduction.
In recent years, there has been concern that the resin shells of coated fertilizers (fertilizer with a resin coating to regulate fertilizer efficacy) are leaking out of paddy fields as an environmental problem. Fertilizer manufacturers have been developing resins with improved disintegrating properties for coating fertilizers. On the other hand, we in the agricultural industry are also investigating the amount of residual resin shells flowing out of the field and examining methods to prevent them from flowing out. In this paper, we introduce our study on the nitrogen leaching characteristics, fertilizer effect, and collapsibility of resin shells when rice plants are grown using a coated fertilizer with new resin with enhanced collapsibility from 2019 to 2021.
Nitrogen leaching characteristics from coated fertilizer
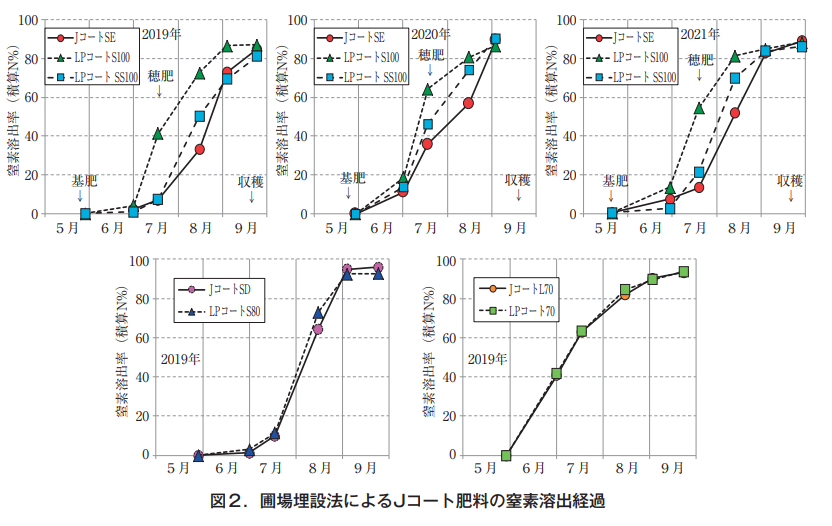
There are two types of nitrogen leaching characteristics of coated fertilizers: linear and sigmoidal types, in addition to long and short leaching days. In rice cultivation in this prefecture, LP Coat S100 (sigmoid type 100-day type) is a representative fertilizer for total basal application, and J Coat SE was tested as an alternative to LP Coat S100 to investigate its leaching characteristics. In addition, J-Coat SD was used as a replacement for LP-Coat S80, and J-Coat L70 was used as a replacement for LP-Coat 70, and their leaching characteristics were also investigated.
Fertilizer and soil were placed in non-woven fabric bags and buried in the field soil, and the bags were periodically collected to wash out the fertilizer and analyze the amount of residual nitrogen. The amount of nitrogen remaining in the bag was analyzed by periodically collecting the bag, washing out the fertilizer, and calculating the amount of nitrogen that had decreased since the beginning of the burial as the leaching rate (Figure 1).

The J-Coat SE used in the cultivation test was initially assumed to have intermediate dissolution characteristics between LP-Coat S100 and SS100. The test results showed that the elution characteristics were similar to those of LP Coat SS100. J-Coat SD and J-Coat L70 had dissolution characteristics similar to those of LP-Coat S80 and LP-Coat 70, respectively, indicating that J-Coat fertilizers have dissolution characteristics equivalent to those of the corresponding existing LP-coated fertilizers (Figure 2).
Growth and yield of paddy rice under total basal fertilizer application
In Nagano Prefecture, climatic conditions and varieties vary from region to region, and in the main variety Koshihikari cultivation area, the use of full basal application of fast-acting + coated fertilizers has increased considerably, replacing the previous system of basal + ear fertilizer application. Although the amount of ear fertilizer varies from region to region, many local brand fertilizers are being used in which the nitrogen content is replaced by a sigmoid-type 100-day fertilizer, and phosphoric acid, potassium, and other elements are blended according to actual conditions. Cultivation trials were conducted in a field plot (Suzaka City) using LP Coat S100 as a control and J Coat SE (Table 1).
Growth in the early to mid-season, which is mainly affected by fast-acting nitrogen, was similar in all test plots. Growth after the early ear formation stage, when the influence of ear fertilizer and cover fertilizer was significant, was similar, and culm length, ear length, ear number, and tillering of J-coated SE were similar to those of the control at the maturity stage (Table 2).
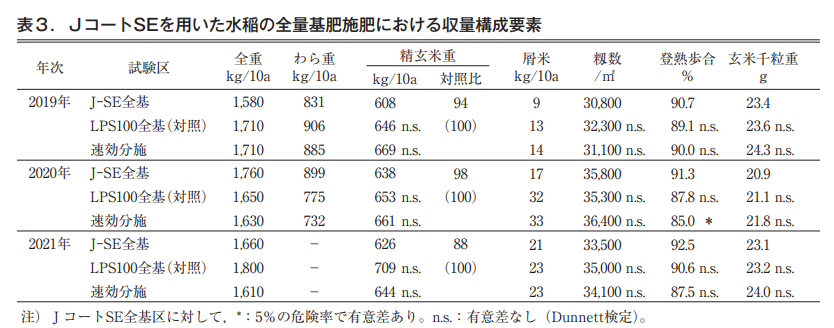
In the paddy rice yield survey, the milled brown rice weight of the J-coated SE plot was slightly lower than that of the control LP-coated S100 plot in some years, but there were no significant differences due to statistical treatment. In all cases, the J-coated SE and LP-coated S100 areas yielded more than 600 kg/10a. In addition, there were no significant differences in yield components between the J-coated SE and the 2020 fast-applied treatments, except for the maturity yield (Table 3).
The total basal fertilizer application for Koshihikari in this prefecture has been warming now that nearly 30 years have passed since the fertilizer application method became widespread. For this reason, there are arguments that LP Coat SS100 is more suitable than LP Coat S100, which has a slightly slower nitrogen leaching rate. Further study is needed to determine whether or not J-coat SE can be substituted for LP-coat S100, including this point.
Brown rice quality in full basal application of paddy rice
Although there were some differences among years and types of quality instruments in the percentage of fine grains, white immature grains, and blue immature grains of polished brown rice, the quality of J-coated SE rice was comparable to that of the control LP-coated S100 rice. The J-coated SE group tended to be slightly superior to the control LP-coated S100 group, although there were some differences depending on the year and the type of quality analyzer. Protein content of polished brown rice was almost 6.5 to 7.0% (under actual moisture conditions), and there was little difference among test sections (Table 4).
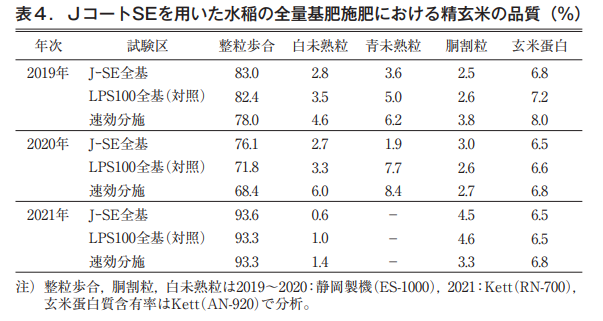
Nitrogen absorption in total basal fertilization of paddy rice
In some years, nitrogen uptake by paddy rice plants in the J-coated SE area was 1 to 2 kg/10a less than that in the control LP-coated S100 area at the time of maturity. However, the amount of nitrogen absorbed from the time of ear formation to maturity, which was mainly derived from the coated fertilizer, tended to be higher than that of the LP-coated S100. This may be due to the fact that, as shown in the nitrogen leaching characteristics of the fertilizer burial test, nitrogen leaching began around the beginning of July for LP Coat S100, whereas it started slightly later for J Coat SE (Table 5).
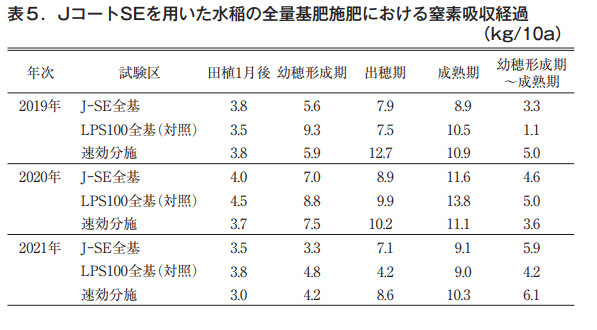
Collapsibility of coated resin shells of J-coat fertilizers
In the first year, fertilizer and soil were placed in non-woven bags and buried in the crop soil in the field in conjunction with rice planting in May 2019, and the coated resin was periodically collected and examined for collapsibility until the harvest season in 2021 (September), two years and four months later. Collapsibility was examined by visual inspection and by the in-sand collapsibility evaluation method, in which the coated resin shell was subjected to a force (Figure 3). The method was developed by ZEN-NOH and conducted by JCAM Agri Co.
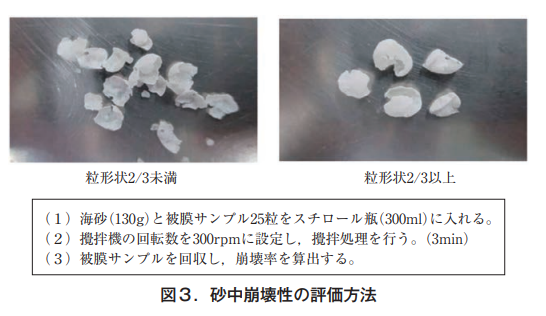
Under the conditions of this test, where the seedlings were buried in the soil, no physical force such as plowing or raking was applied, which is usually done in paddy field operations, and although crushed shells were visually observed, no broken or fragmented shells could be seen until the last survey (Figure 4).
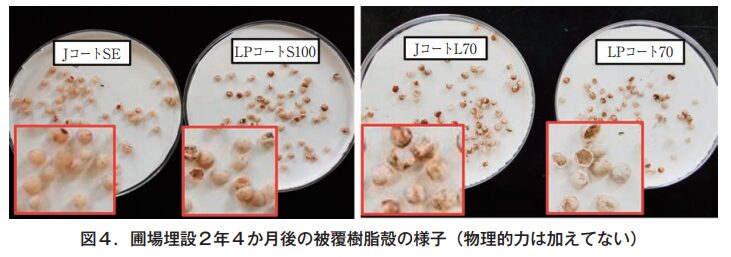
On the other hand, in the second year of the study, the collapse rate (the percentage of the number of grains excluding those that retained at least 2/3 of their original shape; the higher the number, the more advanced the collapse) was 0% for LP Coat 70 and LP Coat S100, while it was 67% for L Coat L70 and 38% for J Coat SE was 38%. When the fertilizers were exposed to sunlight for 2 months after collection (assuming conditions of exposure to the surface of paddy soil), the disintegration rate increased to 83% for LP Coat 70 and 15% for LP Coat S100, indicating that the fertilizers also disintegrated under UV light, which has been shown previously. The disintegration rates of J-Coat L70 and J-Coat SE increased to 93% and 58%, respectively, after exposure to sunlight (Figure 5). The disintegration rate of J-Coat L70, a linear eluting fertilizer, is considered to be higher than that of the sigmoidal eluting fertilizer because of its lower resin content (thinner thickness).
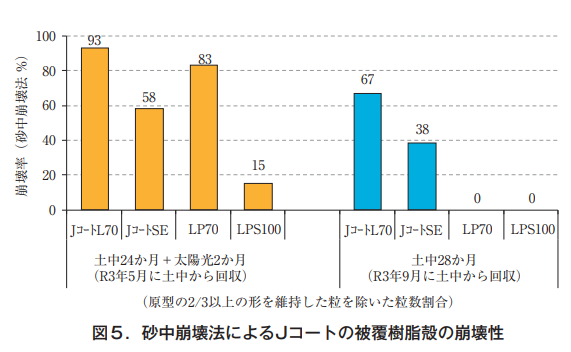
Since the specific gravity of the resin coating of J-coat and LP-coat fertilizers is greater than 1.0, when the resin is broken into several pieces, it is not impregnated with air and does not sink and float in the rice surface water at the time of rice paddling. Therefore, even if the resin shells of the fertilizer do not completely decompose and disappear by the following spring, and even if the rice surface water flows out of the field before rice planting, the amount of shells flowing out is not considered to be large. Therefore, the results of the disintegration test, assuming the conditions of actual physical force such as tilling, indicate that the disintegration rate of J-coat is higher than that of LP-coat, and that sunlight further accelerates the disintegration rate, which may be effective in reducing the amount of runoff out of the paddy fields.
Conclusion
The results of nitrogen leaching characteristics, rice growth, and yield of J-coated fertilizers showed that they are as promising as existing LP-coated fertilizers for total basal application to rice paddies. As for the leakage of coated resin shells out of the paddy field, it is urgently needed to investigate methods to prevent the leakage of non-collapsed fertilizer shells out of the paddy field by arable and physical methods, and the use of J-coated fertilizers with improved collapsibility may have a certain effect.
Coated fertilizers are available in a wide range of slow-release rates, with linear and sigmoidal leaching characteristics, and have a temperature dependence that responds to the weather (growth) of the year to some extent. For this reason, various fertilizer application methods have been developed for rice and many other crops as stable fertilizer with regulated efficacy, contributing greatly to labor saving and cost reduction. By solving the problem of fertilizer shell runoff, which is currently being faced, it is expected to become more widely used in the future.
No Soil - No. 26 Agriculture and Environmental Issues - Part 1
わが国の窒素循環の問題点
前 ジェイカムアグリ株式会社
北海道支店 技術顧問
松中 照夫
The beginning of agriculture was the first human challenge to nature. In the sense of adding human intervention to the natural environment, it can be said to be the beginning of environmental destruction. Later, after the Industrial Revolution, human activities became so active that they altered the natural environment. Agriculture is no exception.
In many countries today, environmental pollution originating from agriculture is a problem. When the amount of nutrients applied to agricultural land exceeds the nutrient holding capacity of the soil, the excess nutrients flow into the groundwater, rivers, and air in the surrounding environment, causing environmental pollution. This month, we will consider the relationship between agriculture and environmental pollution.
The first issue is unique to our country, which has a low food self-sufficiency rate.
Changes in Japan's food self-sufficiency rate
In 1960, the Japanese government made two major cabinet decisions. The first was to accelerate economic growth through the Income Doubling Plan, and the second was to enact the Basic Agricultural Law. In 1960, Japan's food self-sufficiency rate (hereafter referred to as the calorie-based total calorific value of food supply) was 79%. Since then, however, Japan's food self-sufficiency rate has continued to decline, stagnating at 38 to 39% since 2010. The target for food self-sufficiency is 45% by 2030. Achievement of this goal is in jeopardy.
The main reason for the decline in our country's food self-sufficiency rate over the past 60 years has been the need to meet the domestic demand for food, which has been a major factor in the decline in our country's food self-sufficiency rate.
The reason for this is that the basic stance of "food for the family" was not maintained, and furthermore, the consumption pattern of food has changed. For example, a comparison of the 56-year period between FY1965 and FY2021 shows that the total heat supply, which is the sum of the heat supply of each item per person per day, was 2,459 kcal in FY1965 and slightly less than 2,256 kcal in FY2021 (Figure 1).
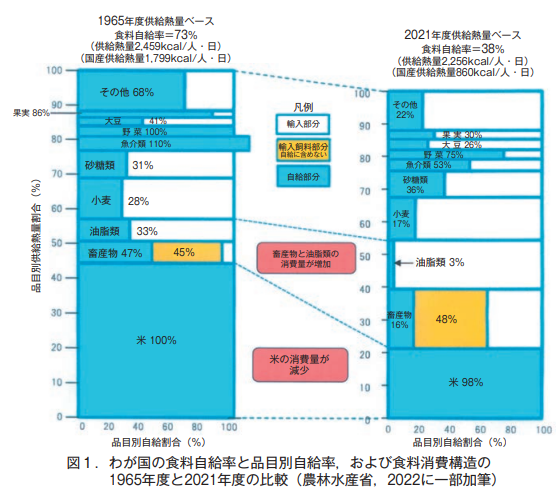
However, the consumption of rice has decreased significantly over the past 56 years (decrease in heat supply), and this decrease has been offset by the increased consumption of livestock products (meat, eggs, dairy products, etc.) and oils and fats (soybean oil, rapeseed oil, etc.) (increase in heat supply) (Figure 1). In addition, although their share of total heat supply has not changed significantly over the past 56 years, the self-sufficiency rate in heat supply has declined significantly for fruits, fish, seafood, wheat, soybeans, vegetables, and "other" (Figure 1).
These changes in food consumption patterns and the decline in self-sufficiency rates by product category are attributable to the shift in the nation's eating habits from the "Japanese style" of rice and fish to the "Western style" of bread, pasta, and dairy and meat products. However, this change in food consumption is unlikely to be the result of a simple change in national preferences or an increase in income. Rather, it should be seen as the result of Japan's policy guidance in response to the U.S. strategy of exporting surplus agricultural products after World War II (Kashiwa, 2012).
Food production, consumption and nitrogen cycle in Japan
The low rate of food self-sufficiency means that nutrients from foreign soil are absorbed by crops, which are then brought into Japan in large quantities as imported food. Nitrogen (N), in particular, tends to have a negative impact on the environment, as we will discuss in the next section. Let us compare the changes in the amount of N circulating in Japan between 1961 and 2005, the years for which data are available (Figure 2).
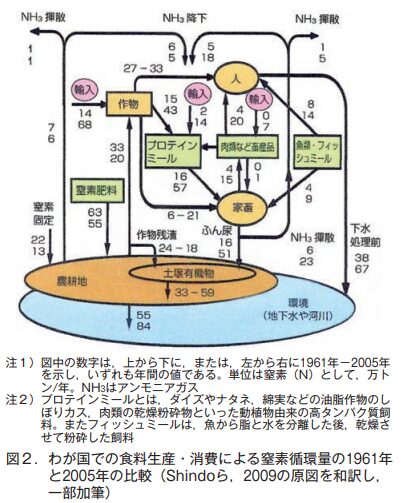
(1) N circulating volume in 1961
食料自給率が78%だった1961年,輸入されたNは,作物から14万トン,プロテインミールから2万トンで,年間わずかに16万トンだった。一方,農耕地に供給されるNは化学肥料由来(年間63万トン)が最も多く,家畜のふん尿由来のN(同16万トン)は,生物的N固定で土にはいる量(同22万トン)より少なかった。飼養されている家畜の頭数が少なかったからである。最終的に1年間で地下水や河川へ流出したNは,農耕地からの55万トンと,人間の排泄物(下水処理前の値)からの38万トンの合計93万トンだった。このほか大気に揮散したN(アンモニアガスとして)は,化学肥料や家畜ふん尿由来の合計で2万トンだった。
(2) Amount of N circulating in 2005
In contrast, in 2005, the population was 128 million, 35% more than in 1961. The food self-sufficiency rate fell to 40%, resulting in a large increase in total imported N, 5.6 times that of 1961, to 890,000 tons (680,000 tons from crops, 140,000 tons from protein meal, and 70,000 tons from livestock products). Conversely, the amount of N entering agricultural land as biological N fixation, chemical fertilizers, and crop residues has decreased. This is because domestic food production has decreased.
Increased consumption of livestock products requires an increase in the number of livestock raised to support their production. The feed for the increased number of livestock is dependent on imports. Therefore, N contained in the imported feed is turned into livestock manure, which flows into agricultural lands. The amount of this inflow was 510,000 tons in 2005, 3.2 times the amount in 1961. Furthermore, the amount of N discharged from human excrement was also 1.8 times that of 1961. This is more than the population growth rate (35%), confirming the high protein content of food.
As a result, it was estimated that in 2005, the amount of N discharged from agricultural lands into groundwater and rivers increased to 840,000 tons per year, and the total of 670,000 tons discharged from human excrement into the environment was added, resulting in an annual discharge of 1.51 million tons into groundwater and rivers. This amount is 1.6 times that of 1961. The amount of N volatilized as ammonia gas from fertilizers and livestock manure into the atmosphere was 60,000 tons, three times the 1961 level.
Japan's low food self-sufficiency rate and changes in food consumption trends have greatly increased the amount of N brought in from overseas. This has a great potential to cause water pollution of groundwater and rivers in Japan, as well as deterioration of the atmospheric environment (Shindo et al., 2009). This is truly a problem caused by the low self-sufficiency rate. In the next issue, we will consider the specific environmental pollution sources of N released into the environment.
次回は,この環境に流出するNが具体的にどのような環境汚染源となっているかを考える。