

Site Search
Search within product
第729号 2021 (R03) .04発行
Click here for PDF version
農業と科学 2021/04

本号の内容
§湛水条件下の水田土壌の
可給態リン酸の温度反応とその推定
東北大学大学院 農学研究科
Field Science Center for Ecological Field Education and Research
教授 西田 瑞彦
§気候変動緩和と肥効調節型肥料
千葉大学大学院 園芸学研究院
名誉教授 犬伏 和之
of paddy soils under waterlogged conditions.
可給態リン酸の温度反応とその推定
東北大学大学院 農学研究科
Field Science Center for Ecological Field Education and Research
教授 西田 瑞彦
Introduction
According to the U.S. Geological Survey, the world's economic reserves of phosphate ore are 69 billion tons, which is approximately 290 times the 240 million tons produced in a single year in 2019 (U.S. Geological Survey 2020). Economic reserves fluctuate with the depletion of ore deposits, the discovery of new deposits, and increases in the amount that can be mined due to technological advances, and are expected to be mined economically for a longer period of time than was once thought possible. However, it is certain that the amount of reserves is limited. In addition, reserves are unevenly distributed in some parts of the world, with large reserves in North Africa, the Middle East, China, etc. Therefore, depending on the situation in these regions, the amount of reserves may be limited. Therefore, the supply may become unstable depending on the situation in these regions. The price of phosphate ore rose sharply in 2008 and is currently lower than that.
However, it has remained higher than before the sharp rise (Figure 1).
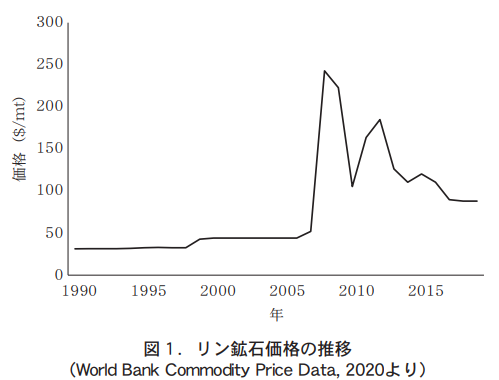
In Japan, the impact of the phosphate fertilizer imports is significant because the country relies entirely on imports, and the need for efficient use of phosphate fertilizer was strongly recognized when the price of phosphate ore rose sharply. As a result, the possibility of fertilizer reduction based on soil diagnosis was studied on a nationwide scale. At the same time, studies were conducted on the variation of soluble phosphoric acid in soil. Here, we introduce a study by the authors (Nishida et al., 2018) on the relationship between temperature and changes in soluble phosphate in paddy soils under waterlogged conditions. This study was conducted at the Daisen Research Station, Tohoku Agricultural Research Center, National Agricultural Research Institute, Japan.
2. relationship between soluble phosphoric acid and integrated temperature under waterlogged conditions
In paddy fields, phosphate deficiency is particularly problematic in the early growth period when the root system is not fully developed. Typical symptoms of phosphate deficiency are reduced offshooting, which leads to a lack of ear number and reduced crop yields. Because the deficiency symptoms are enhanced by low temperatures, cold-weather soils require higher levels of available phosphate than warm-weather soils. Shiga and Yamaguchi (1976) found that 22 mg/100 g of Bray No.2 phosphate is sufficient in a normal year, but 40 to 50 mg/100 g or more is required in a cold year to prevent yield loss with no phosphate fertilizer application in cold regions.
This suggests that the relationship between soil phosphate availability and temperature under waterlogged conditions during the paddy rice growing season is particularly important under cool weather conditions. Therefore, we collected paddy soil samples from all over Japan (Akita, Iwate, Yamagata, Miyagi, Tochigi, Ibaraki, Niigata, Aichi, Okayama, Fukuoka, Miyazaki, and Kagoshima) and incubated them under waterlogging conditions at 10°C, 17.5°C, and 25°C in moist soil to investigate the relationship between temperature and soluble phosphate under waterlogging conditions.
Figure 2 shows the relationship between the total temperature and the availability of phosphoric acid (Bray No. 2 phosphoric acid) under waterlogged conditions for several soils. The relationship between total temperature and Bray No. 2 phosphate was similar at different incubation temperatures. Therefore, all subsequent analyses were based on the integrated temperature without distinguishing the incubation temperature conditions. The values of Bray No. 2 phosphate under waterlogged conditions and their variation with integrated temperature differed among the soils. In many soils, Bray No. 2 phosphate tended to increase with increasing integrated temperature, while in others it remained almost unchanged.
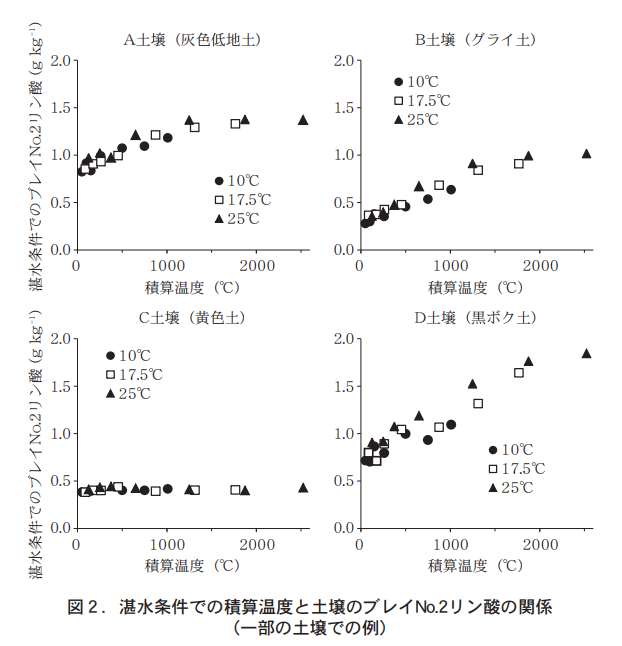
3.Estimation of the relationship between soluble phosphoric acid and accumulated temperature under waterlogged conditions at the early stage of rice growth.
As mentioned earlier, phosphate deficiency in paddy fields becomes a problem in the early stage of growth. In Daisen City, Akita Prefecture, a cold region, the accumulated soil temperature (5 cm deep in the ground) in the paddy field during the month (May 20 to June 20) just before the transplanting and drying out was 650°C on average over the four years. Bray No. 2 phosphate increased almost linearly under waterlogging conditions up to the integrated temperature of 650°C (Figure 2). Therefore, we considered that these relationships could be expressed as a straight line and performed a regression analysis. As a result, significant positive correlations were found except for four soils where Bray No.2 phosphate did not change significantly with increasing integrated temperature (data omitted).
Therefore, it was thought that the changes in Bray No. 2 phosphate up to an integrated temperature of 650°C could be expressed by a first-order regression equation specific to each soil. However, to obtain this soil-specific regression equation, a waterlogging experiment with moist soil is necessary. Since this would require time and labor, it would be desirable to be able to estimate the change in Bray No. 2 phosphoric acid under these waterlogged conditions using the analysis of air-dried soil. Therefore, we next examined the possibility of estimating this regression equation using the analyzed values of air-dried soil.
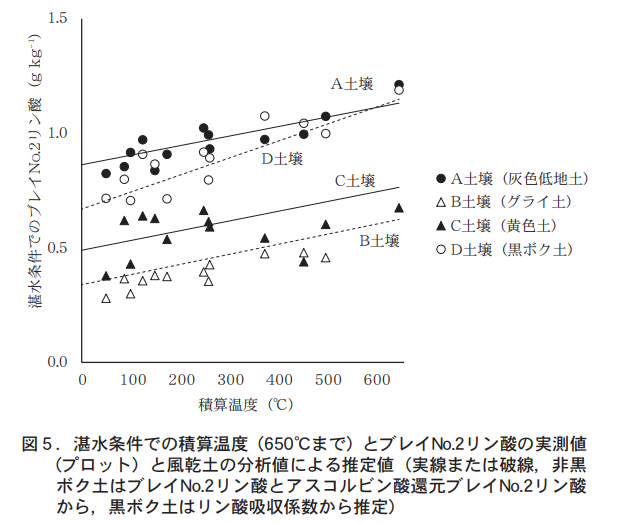
We first examined the intercept of the regression equation for each soil, i.e., the relationship between the initial value of Bray No. 2 phosphoric acid after waterlogging and the analyzed value using air-dried soil (Table 1). Subsequent analyses were conducted separately for the non-black-bok soils (15 gray lowland soils, 3 gley soils, 1 gray plateau soil, and 1 yellow soil) and the black-bok soils (6 soils). The strongest correlation was found between Bray No. 2 phosphate (R2 = 0.947*) in the air-dried soils and the phosphate absorption coefficient (R2 = 0.920) and the intercept of the regression equation in the non-black soil (Table 1, Figure 3). These results suggest that the initial value of Bray No. 2 phosphate after waterlogging (intercept of the regression equation) can be estimated by the Bray No. 2 phosphate of air-dried soil in the non-black soil and by the phosphate absorption coefficient in the black soil.
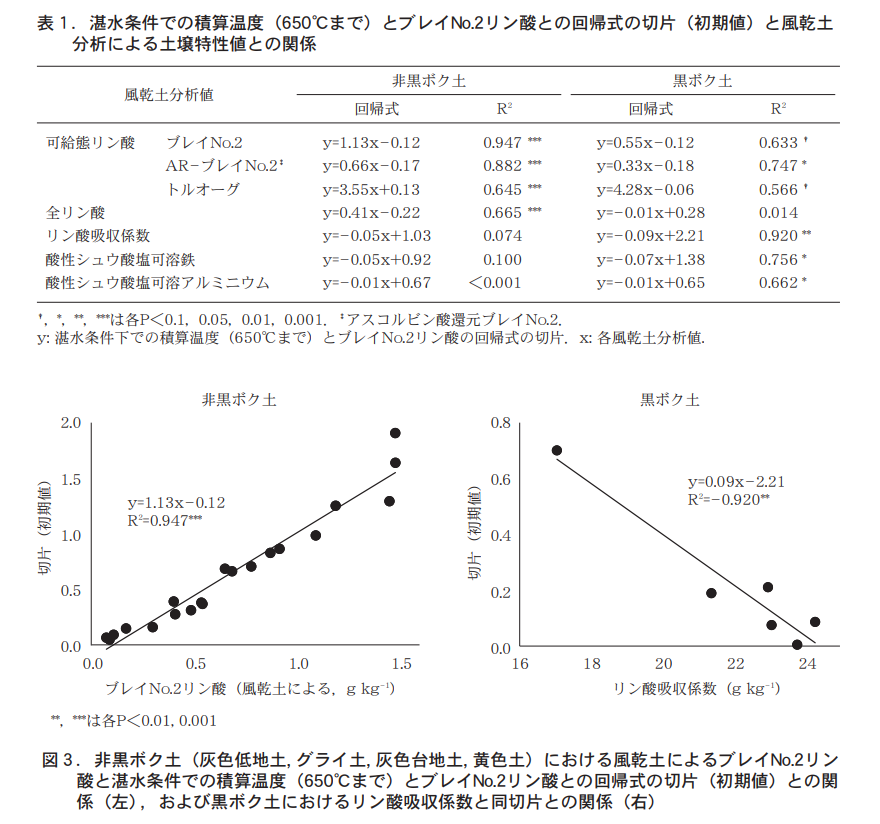
It is not difficult to understand that the initial value of Bray No. 2 phosphate after waterlogging wet soil can be estimated by the Bray No. 2 phosphate value of air-dried soil. However, a strong negative correlation was observed between the initial value of Bray No. 2 phosphate after waterlogging and the phosphate absorption coefficient in the black granite soil. This is interesting because it indicates that the ability to adsorb and fix phosphoric acid determines the initial value of Bray No.2 phosphoric acid after waterlogging, rather than the ability to supply phosphoric acid, which is evaluated by the availability of air-dried phosphoric acid.
Next, the degree of increase in Bray No. 2 phosphoric acid (slope of the regression equation) with respect to the integrated temperature under waterlogged conditions was examined (Table 2). The difference between ascorbic acid reduced Bray No. 2 phosphate and Bray No. 2 phosphate for both non-black soil and black soil (non-black soil: R2
=0.826, R2 =0.847), or the difference between ascorbic acid-reduced Bray No. 2 phosphate and toluoglinic acid (non-black soil: R2 =0.830, black soil: R2 =0.739*) (Table 2, Figure 4). This is in contrast to the difference between soluble phosphoric acid (ascorbic acid-reduced Bray No. 2 phosphoric acid) under reducing conditions and non-reducing conditions.
The difference between the ratable phosphoric acid (Bray No. 2 phosphoric acid and toluogue phosphoric acid) and the Bray No. 2 phosphoric acid under waterlogging conditions indicates that the degree of increase of Bray No. 2 phosphoric acid with respect to the integrated temperature can be estimated.
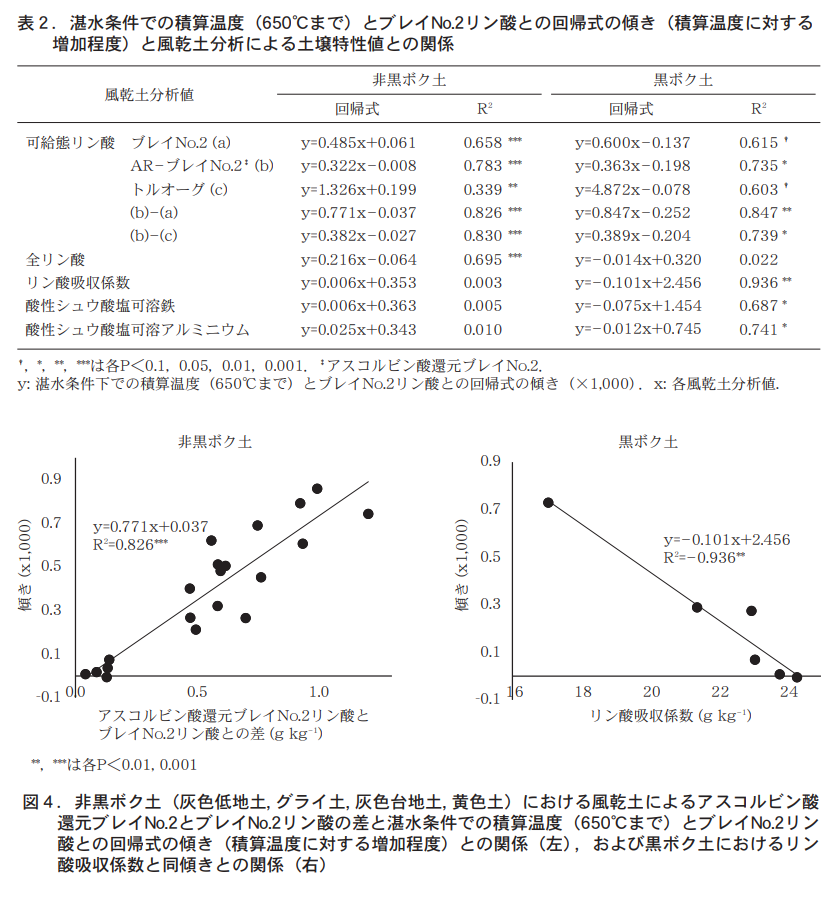
On the other hand, the highest coefficient of determination in the black granite soil was the phosphate absorption coefficient (R2 = 0.936), which showed a strong negative correlation (Table 2, Figure 4). This indicates that in addition to the ability to supply phosphoric acid, the ability to adsorb and fix phosphoric acid strongly affects the extent to which Bray No. 2 phosphoric acid increases with temperature under waterlogging conditions in the black granite soil. In other words, the initial value of Bray No.2 phosphate under waterlogging conditions and its subsequent change with accumulated temperature are strongly limited by the ability to adsorb and fix phosphoric acid in the black granite soil.
The above results suggest that the changes in Bray No.2 phosphoric acid and its total temperature in cold climates under waterlogging conditions about one month after transplanting can be estimated from the availability of air-dried soil phosphate (Bray No.2 phosphoric acid and ascorbate-reduced Bray No.2 phosphoric acid) in the case of non-black soil and from the phosphate absorption coefficient of air-dried soil or availability of Bray No.2 phosphoric acid (Bray No.2 phosphoric acid and ascorbate-reduced Bray No.2 phosphoric acid) in the case of black soil. Phosphate (Bray No.2 phosphate and ascorbate-reduced Bray No.2 phosphate) for black granite soils. Examples of estimations for several soils are shown in Figure 5.
4. Conclusion
In this study, the response of soil soluble phosphoric acid to temperature under waterlogged conditions was determined for a wide range of soils. In this study, the Bray No. 2 method (quasi-method) was used to analyze the soluble phosphoric acid in soils under waterlogged conditions. The solid-liquid ratio of extraction was 1:20 according to the Soil Environmental Analysis Method. However, the basic target values for improvement of paddy soil in the basic guidelines for soil fertility enhancement and the diagnostic standard values at the prefectural level are almost always set using the Tor-Og method. In a previous study by Shiga and Yamaguchi (1976), the solids/liquid ratio was 1:10 and the Bray No. 2 method was used for analysis of free-form phosphoric acid.
When more effective use of phosphorus resources is strongly required and more precise soil diagnostic standard values are needed, it will be effective to clarify the relationship between analytical values by each method and to integrate these findings.
thanks
The authors would like to express their deepest gratitude to the late Dr. Koji Yoshida, who conducted this research with us and provided guidance and encouragement throughout. The authors would like to express their gratitude to the agricultural research institutes in each prefecture, the Kyushu-Okinawa Agricultural Research Center of the National Institute of Agrobiological Sciences, and Tohoku University for their cooperation in soil sampling for this study.
References
●Mizuhiko Nishida, Koji Yoshida, Tomoki Takahashi. 2018.
Estimation of changes in available soil phosphate under submerged
conditions associated with temperature during the tillering stage of rice plant
in the cool climate region of Japan.
Communications in Soil Science and Plant Analysis. 49. 1695-1706.
●志賀一一, 山口紀子.1976.
寒地稲作における土壌の燐酸肥沃度及び燐酸施肥の効果に関する研究 第3報
窒素施用量及び年次変動との関係. 北海道農業試験場研究報告. 116. 139-155.
●U.S. Geological Survey. 2020.
Mineral Commodity Summaries.https://www.usgs.gov/centers/nmic/phosphate-rock-statistics-and-information
Climate Change Mitigation and Fertilizer Regulation
千葉大学大学院 園芸学研究院
名誉教授 犬伏 和之
Introduction
It is still fresh in our minds that Japan has decided to become carbon neutral by 2050 in order to prevent global warming, and this goal is also appropriate worldwide in order to minimize the effects of climate change. In the post-Corona period, Europe is also aiming for a more recycling-oriented society under the banner of "Green Recovery. The reduction of greenhouse gas emissions from agricultural activities has already been introduced in this journal1) , and this paper introduces some examples from Asia, especially the release of nitrous oxide (N2O), which has a high greenhouse effect, from agricultural lands and the effects of fertilizer with regulated fertilizer efficacy for its control.
2. global balance and production of dinitrogen monoxide (N2O)
The Global Carbon Project (GCP) has published "Global Nitrous Oxide Balance 2020," a global N2O balance that details all sources and sinks of nitrous oxide (N2O). A paper summarizing the results of this research was published in the international journal Nature2) on October 8, 2020, and an online public forum was held on October 29, 2020, jointly hosted by the GCP Tsukuba International Office, the Global Environmental Research Center, JAMSTEC, and Future Earth Japan Hub. The forum was held on October 29, 2012. In this forum, the co-authors of the paper, especially lecturers specializing in agriculture, food production, soils, and fertilizers, provided detailed explanations, and the author reported on the influencing factors on N2O release and N2O mitigation measures under the title of "N2O generation process and feasible mitigation from the soil perspective. The author also reported on the influencing factors on N2O release and N2O mitigation measures.
First, Akihiko Ito, Director of the Material Cycle Modeling and Analysis Laboratory, Global Environment Research Center, presented an overview of the global N2O budget (2007-2016) (Figure 1), showing that N2O emissions are of natural (57%) and anthropogenic (43%) origin, with natural emissions amounting to 9.3 Tg (1Tg = 1012 g, or 1 million tons) and 7.3 Tg of anthropogenic emissions. In total, about 17 Tg is released every year.
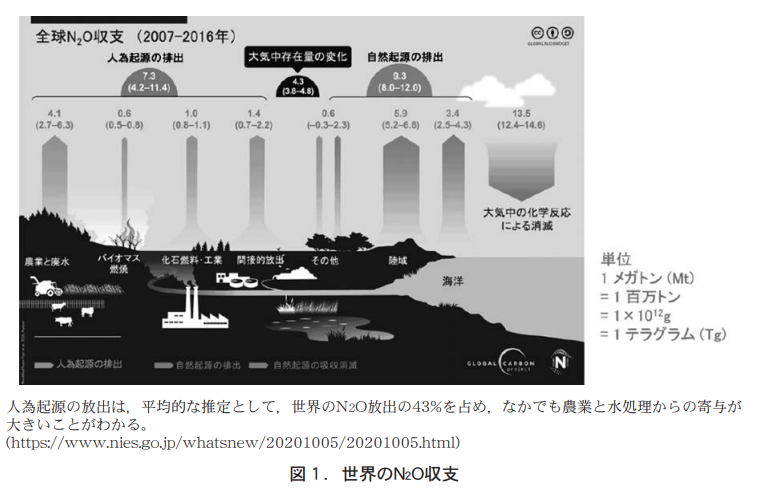
On the other hand, 13.5 Tg is dissipated annually due to chemical reactions in the stratosphere and troposphere. Subtracting this amount, 3.5 Tg remains in the atmosphere each year. By region, Africa is the largest emitter (about 3 Tg/year). This is due to the large area of Africa and the large amount of N2O emitted from tropical forests. Tropical forests have high temperatures and high precipitation throughout the year, which is thought to increase microbial activity and N2O release. Next are South America and East Asia, with annual N2O emissions of approximately 2 Tg. South America, like Africa, releases most of its N2O from tropical forests, while East Asia's N2O emissions from agriculture are prominent. The oceans also emit 2 to 3 Tg per year, but there are differences in estimation methods, and uncertainty remains.
Global N2O emissions are increasing by more than 11 TP3T annually. Agriculture is the largest source of anthropogenic emissions. Regional variations in anthropogenic emissions show that in recent years, emissions have increased in Asia, followed by South America and Africa, where agricultural activities are more active. In particular, the increase in East Asia is related to the increase in chemical fertilizer and manure inputs (plus direct emissions) to agricultural land, which have more than doubled over the past 30 years.
3.N2O formation and influencing factors
The author then introduced the process of N2O formation, stating that it can be a byproduct of nitrification under aerobic conditions (ammonia is oxidized by microorganisms to nitrite and nitrate) or an intermediate product of denitrification under anaerobic conditions (nitrate nitrogen in soil is reduced and released as molecular nitrogen into the atmosphere), as in paddy fields. In the case of rice paddies, it is the intermediate product of denitrification under anaerobic conditions.
Factors affecting N2O generation include: 1. soil type, 2. additives (biochar, etc.), 3. soil management and control (slow-release fertilizers, nitrification inhibitors, etc.), 4. water management in paddy fields, and the relationship with methane generation control (trade-off between methane and N2O). Due to space limitations, this paper will focus on 3. and 4.
4. soil management and control of N2O release
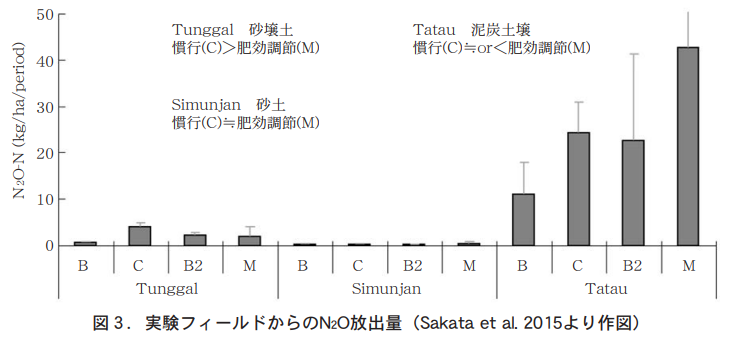
In recent years, with the development of tropical forests and the rapid increase in oil palm plantations as a raw material for vegetable oils and fats, we conducted a field survey at large-scale plantations in one site in Indonesia (Tunggal, Fig. 2 (a)) and two sites in Malaysia (Simunjan, Fig. 2 (b); Tatau, Fig. 2 (c)), using inorganic soil with low organic matter (a), (b) The test plots were located in two large farms (Simunjan, Fig. 2 (2); Tatau, Fig. 2 (3)) on inorganic soil with low organic matter (1) and peat soil with high organic matter (3), and were divided into: 1) no fertilizer application (B), 2) conventional application with surface application of chemical fertilizer at conventional amounts (C), 3) only partial tillage (B2), 4) a test plot with half the fertilizer amount (Jaycam Agri, MEISTER (LP coat)) applied after partial tillage (M). N2O release was measured for 340 to 580 days and compared3) .
From Tunggal, N2O release was significantly lower with the regulated fertilizer (Figure 3), while Simunjan is a rainy area, so the fertilizer flowed away and less was released. The oil palm yield was almost the same with half the amount of fertilizer as with the regulated fertilizer4) , indicating that the loss was small and N2O release was also reduced. On the other hand, N2O release was an order of magnitude higher in Tatau, a peat soil, and the effect of fertilizer was not clear. The effects of fertilizers on tropical soils varied greatly depending on the type of soil.
Another promising mitigation measure is nitrification inhibitors. The nitrification process involves oxidation of ammonia and oxidation of nitrite, and inhibitors have been developed to suppress N2O release by blocking these processes, and synthetic chemicals such as dicyandiamide (DCD) are already being used in some fertilizers. Plant-derived nitrification inhibitors being developed at Makassar University and the International Research Center for Agriculture, Forestry and Fisheries in Indonesia can considerably suppress N2O release5) . It is expected to be put to practical use in the future.
5. water management in rice paddy fields and its competitive relationship with methanogenesis
To reduce methane, a greenhouse gas emitted from rice paddies, by changing water management methods, we conducted a field experiment in collaboration with Tamil Nadu Agricultural University in southern India to confirm the effectiveness of such methods overseas (Figure 4).6) In the high-yield rice cultivation (SRI) area, where the groundwater level was lowered to about 15 cm every few days by intermittent irrigation combined with sparse planting of single seedlings, methane emissions were reduced by 40-50% compared to the control area, which was constantly flooded, saving about 501 TP3 T of water.

Furthermore, there was no significant change in N2O emissions, which had been a concern because of the trade-off relationship between methane reduction and N2O emissions. Furthermore, similar water-saving cultivation tests were conducted in the Philippines, Indonesia, Thailand, and Vietnam in Southeast Asia, and it was confirmed that methane was reduced and N2O emissions were not significantly affected.
6. at the end
The following is a paper by Dr. Rattan Lal, past president of the International Union of Soil Science (IUSS)7) on the current impact of the new corona. Soil health is the foundation of people's health, poverty alleviation, and climate change countermeasures. In the future, human activities are expected to be transformed by COVID-19, which will change food production systems, and soil management methods will be transformed to place more emphasis on sustainability than ever before. Fertilizer production and utilization will also be affected. The atmospheric lifetime of N2O is about 120 years, which is one order of magnitude longer than other greenhouse gases, so we should promote reduction measures to avoid a negative legacy for the future.
References
1)斎藤雅典:2019.
農業活動と温室効果ガス排出削減,
農業と科学,令和元年12月号,7−11.
2)Tian H., Xu R., Canadell J.G., Yao Y. et al.:2020.
A comprehensive quantification of global nitrous oxide sources and sinks,
Nature, 586, 248−256.
3)Sakata R., Shimada S., Arai H., Yoshioka N., Yoshioka R., Aoki H., Kimoto N.,
Sakamoto A.,Melling L., and Inubushi K.:2015.
Effect of soil types and nitrogen fertilizer on nitrous oxide and carbon
dioxide emissions in oil palm plantations, Soil Science and Plant Nutrition, 61, 48−60.
4)Sakata R.:2017.
Soil, fertilizer and topography affect emissions of nitrous oxide and carbon
dioxide, and yield of oil palm in Indonesia and Malaysia, 千葉大学博士論文.
5)Jumadi O., Hala Y., Iriany R.N., Makkulawu A.T., Baba J., Hartono, Hiola St.F.,
and Inubushi K.: 2020. Combined effects of nitrification inhibitor
and zeolite on greenhouse gas fluxes and corn growth. Environmental Science
and Pollution Research, 27, 2087−2095.
6)Oo A.Z., Sudo S., Inubushi K., Mano M., Yamamoto A., Ono K., Osawa T.,
Hayashida S., Patra P.K., Terao Y., Elayakumar P., Vanitha K., Umamageswari C.,
Jothimani P. and Ravi V.:2018. Methane and nitrous oxide emissions
from conventional and modified rice cultivation systems in South India,
Agriculture, Ecosystems and Environment,252, 148−158.
7)Lal R.:2020. Soil Science beyond COVID-19,
Journal of Soil and Water Conservation, 1−3. doi:10.2489/jswc.2020.0408A