

Site Search
Search within product
別冊特集号 2025(R7).07発行
Click here for PDF version
「土のはなし」別冊特集号によせて
前 ジェイカムアグリ株式会社 技術顧問
松中 照夫
本誌で4年間連載された「土のはなし」がこの度,特集号として1冊にまとめられた。編集部のご厚意に心から感謝したい。1冊にまとめるにあたり,「土のはなし」の内容を7つのテーマに整理し,章立てした。
第1章は「作物にとってよい土であるための条件」である。「土づくり」で目指すべき土を具体化するため,よい土であるための4つの条件を,数値目標を含めて提示し,解説した。第2章は「堆肥と化学肥料」。堆肥の有効利用と,とかく誤解されがちな化学肥料を正しく理解してもらうための情報を提供した。第3章「植物の養分吸収と吸収された養分の植物体内での働き」は,植物の養分吸収のしくみや,作物の品質に関わる窒素の働きを述べている。第4章「土のでき方と地球上での役割」では,土が地球上の生命を育み,その土は環境がつくることを論じた。第5章「農業に起因する環境問題」は,農業という人間活動,中でも農地に与えた窒素の環境への悪影響を紹介した。第6章「劣化する世界の土」は,土の劣化の最大要因が不適切な人間活動であることを話した。第7章「有機農業と慣行農業」では両者の優劣を論じるのではなく,それぞれの養分源の課題を指摘した。連載最終回では,老齢化するわが国農業の将来を考えた。この連載記事40回を通して,私の土への思いを読者の皆さんが読み取っていただけるなら,この上ない喜びである。
ご愛読いただいた皆様に改めて感謝致します。毎回の原稿を点検していただいたご校閲者の方々,執筆を激励して下さった編集部の皆さん,ありがとうございました。
目 次
第1章 作物にとってよい土であるための条件
第 1 回 よい土とはどんな土か
第 2 回 よい土の条件 物理的性質−その1 根を支える土の厚み
第 3 回 よい土の条件 物理的性質−その2 土の硬さはどのようにしてきまるのか
第 4 回 よい土の条件 物理的性質−その3 断面でわかる排水の良否
第 5 回 よい土の条件 物理的性質−その4 適度に水を保持し排水もよい土とは
第 6 回 よい土の条件 化学的性質−その1 土の酸性度(pH)
第 7 回 よい土の条件 化学的性質−その2 酸性障害がでる土とでにくい土
第 8 回 よい土の条件 化学的性質−その3 適度に含まれる作物の養分
第 9 回 よい土の条件 化学的性質−その4 土が養分を保持するしくみ
第10回 よい土の条件のまとめ−どんな土でも必ずよくなる−
第2章 堆肥と化学肥料
第11回 堆肥は養分移転資材として登場した−養分の補給方法を考える−
第12回 堆肥の効果の現れ方と土の条件−土の黒さが決め手−
第13回 有機物資材の種類とその効果−C/N比が要点−
第14回 養分源が堆肥から化学肥料へ変化する時代−その歴史的経緯−
第15回 化学肥料だけしか使わない畑のコムギの生育−堆肥だけの畑と比べる−
第16回 堆肥と化学肥料,その効果を比べる−共通点とちがいは何か−
第3章 植物の養分吸収と吸収された養分の植物体内での働き
第17回 植物が水と養分を吸収するしくみ−必要な物質を吸収し,不要な物質は排除する−
第18回 植物が難溶性物質を吸収するしくみ−根から溶解を助ける物質を分泌する−
第19回 吸収された窒素がタンパク質になるまで−植物は必要なアミノ酸をすべて自給する−
第20回 農産物のおいしさに影響するタンパク質と炭水化物はトレードオフの関係
第4章 土のでき方と地球上での役割
第21回 「土は生きている」といわれるのはなぜ?−土は生き物なのか
第22回 地球上の生命を育み,地球環境を保全する土の役割
第23回 原始地球に土はなかった−こうして地球に土が誕生した
第24回 土は環境の産物である−風化と生物の作用が岩石から土をつくる
第5章 農業に起因する環境問題
第25回 農業が環境破壊の始まり−人間活動と環境との関わり−
第26回 農業と環境問題−その1 わが国の窒素循環の問題点
第27回 農業と環境問題−その2 農地由来の窒素による水質汚濁
第28回 農業と環境問題−その3 農地由来の窒素による大気汚染 アンモニア揮散
第29回 農業と環境問題−その4 農地由来の窒素による大気汚染 一酸化二窒素排出
第30回 農業と環境問題−その5 農業由来の温室効果ガスと地球温暖化
第6章 劣化する世界の土
第31回 危機に瀕する世界の土−その1 古代文明の崩壊と土の劣化
第32回 危機に瀕する世界の土−その2 不適切な人間活動が土を劣化させる
第33回 危機に瀕する世界の土−その3 塩類集積による土の劣化とそのリスク
第34回 危機に瀕する世界の土−その4 侵食による土の劣化
第35回 危機に瀕する世界の土−その5 酸性雨による土の劣化
第36回 先進国経済が途上国の土や資源を収奪する−その現実と環境破壊の事例から学ぶこと−
第7章 有機農業と慣行農業
第37回 農地は作物を栽培する土地である−農地で生物の多様性をどう考える−
第38回 有機農業の養分源・堆肥生産の課題−堆肥生産には労力と土地が不可欠−
第39回 慣行農業の養分源・化学肥料の課題−原料の資源枯渇や生産のエネルギー問題−
連載のおわりに
第40回 わが国農業者の高齢化は食料生産への不安要因−高齢化歯止めの鍵は新規参入者支援−
−第1章− 作物にとってよい土であるための条件
第1回 よい土とはどんな土か
令和3 (2021) 年 5月号 (第730号)
今月号から,「土のはなし」としてしばらく連載させていただくことになった。本誌の他の記事のような技術情報とはやや趣を異にし,土にまつわる情報を読み物風に提供したいと思う。
まずは,農業関係者がしばしば語る「よい土」とはいったいどんな土なのか,それを話題に取り上げる。
1. experienced chefs call it "good soil" just by looking at it...
かれこれ10年くらい前の春,北海道ニセコ・羊蹄山麓の農場を,札幌の有名ホテルのベテランシェフと共に訪問したことがある。ジャガイモの植え付けをしているところだった。テレビのレポータがその畑をみてどう思うかと私達に問いかけてきた。そのベテランシェフは,即座に「この畑で取れるジャガイモは美味しいに決まっています。この黒々とした軟らかな土を見ればわかるでしょ。むせかえるこの土の匂いは,ゆでると粉がふくホクホクのジャガイモがとれることを約束しています。」と語ったのだ。その場にいた私には,土を見ただけでいとも簡単に「美味しいジャガイモがとれる」といえるのが不思議だった。
I was happy that the chef considered the soil important enough to determine the taste of the potatoes. However, I cannot for the life of me believe that "black, soft, good-looking soil" determines the taste and yield of potatoes. If it were determined by appearance, we wouldn't have to bother with labor-intensive cross-sectional soil surveys and soil diagnostics.
2. Is soil with a lot of compost or soil with many earthworms "good soil"?
Whenever I ask farmers what kind of soil is "good soil," a phrase is bound to come up. They say things like, "Good soil is soil that has been given plenty of compost," or "You can tell if it is good soil by the number of earthworms it has.
If a "good soil" can be produced by giving plenty of compost, then there is no need for livestock farmers to produce an excess of compost. Even livestock farmers may have farmland, and if they give as much as they can, they will have "good soil. The question is, how much is "plenty" in the phrase "give plenty of manure"? The same is true for "whether there are many earthworms or not. Even if the soil is good if there are many earthworms, how much is "plenty"? Without a clear answer, it is impossible to judge whether the soil is good or bad.
There is nothing inherently good or bad about soil.
19世紀ころまで,「土は地殻の表面を覆う細かく砕かれた岩石からなるやわらかな物体」という程度の理解であった。この考え方に異を唱えたのが,ロシアの若き地質学者ドクチャーエフ(1846〜1903)だった。彼はロシアの大地を北から南までおよそ1万kmも踏破し,土の断面を観察した。そして,寒い地方や暖かい地方,それぞれに特徴ある土の断面ができていることに気づいた。
He believed that soil is formed by the interaction of various factors, such as the type of rock from which it is made, the climate, flora, fauna, and topography of the site, and that the soil changes over time. Given certain environmental conditions, the same soil will be formed if the raw materials are the same. However, even if the raw materials are the same, the soil produced will be different if the environmental conditions are different. This is because the action of living organisms, which play an important role in soil formation, differs greatly depending on the environment. Dochkachev realized that the environment creates soil.
According to Dochtayev's view, we cannot make a value judgment about the soil produced in a given environment as good or bad. This is because the soil produced in a given environment is the only soil that can be produced in that environment.
On the other hand, we who are involved in agriculture judge "good soil" or "bad soil" based on the implicit premise that crops are to be cultivated and on the criterion of whether or not crops can be cultivated without inhibiting their growth in response to that premise. If the criteria are different, the soil may be "good soil" for agriculture, but "bad soil" for the foundation of a large condominium building. Soil itself is not good or bad. It is merely a value judgment made by people.
What kind of soil, then, is soil that does not inhibit the growth of crops, that is, soil that is good for crop production?
4. 4 conditions for good soil for crop production
それは,次の①から④の4条件を満たすことができる土であると私は考えている。すなわち,
①作物の根を確実に支えることができるように,厚く軟らかな土が十分にあること,②適度に水分を保ち,なおかつ適度に排水がよいこと,③土が極端な酸性やアルカリ性を示さないこと,④作物に必要な養分を適度に含んでいること,である。ここで①と②は土の物理的な性質に関わる条件,③と④は土の化学的な性質に関わる条件である。
However, since the above description does not provide specific numerical information to serve as indicators, it is not possible to determine whether each of the conditions is satisfied or not. Table 1 summarizes the four conditions for good soil for crop production, including the specific indicators.
We plan to explain each of these conditions and indicators in the next and subsequent issues. We hope you will look forward to it.

5. concept of biological properties of soil
By the way, when I present the above four conditions, I am often asked why they do not include conditions on the biological properties of the soil (from microbes to animals). Why is it that these four conditions do not include the biological properties of the soil (from microorganisms to animals)? It is not easy to fully explain why in the limited space of a paper. However, we can at least point out the following
All of the above four conditions for good soil have a significant impact on the life of organisms in the soil. However, we do not believe that the fulfillment of these four conditions has a negative impact on the life of organisms in the soil. For this reason, we have not added the biological condition of the soil to the conditions for good soil.
−第1章− 作物にとってよい土であるための条件
第2回 よい土の条件 物理的性質−その1
根を支える土の厚み
令和3 (2021) 年 6月号 (第731号)
In last month's issue, we presented four conditions and their target values for good soil for crop production. From this month onward, each of the four conditions will be explained. In this issue, we will focus on the first condition, "soil thickness and hardness," which is one of the conditions related to the physical properties of soil, and consider its thickness.
First, let's dig up the soil.
In last month's issue, I mentioned that a veteran chef at a famous hotel in Sapporo remarked that he could harvest many delicious potatoes just by looking at the soil. However, I do not have the courage to say such a thing. I don't know what is inside the soil where the roots of the crops grow, even if I stand in the field where the crops are cultivated (the same is true in a home garden) and look at the soil from above. You cannot know what is inside the soil unless you dig it up. First of all, I would like you to choose an area where the crop growth is average in the field and dig the soil by yourself with a shovel.
掘る深さは1mが目安。太陽を背中に受けるようにして掘る(図1) 。ただなんとなく掘るのではなく,自分に対して正面となったところは,土の表面から垂直に壁状にすることに気をつける。
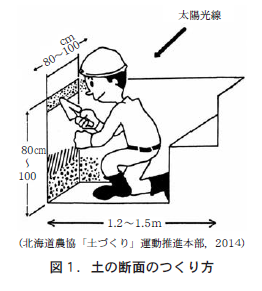
この壁状となったところを土の「断面」という。
When you are digging with a shovel, I want you to remember the hardness of the soil and the feel of the shovel. The reason is that whether you can dig soil easily or not is related to the condition of "soil hardness," which will be discussed in the next article. If the soil becomes too hard to dig, or if it becomes too hard to dig because of rocks (Figure 2), the digging will be terminated.

2. soil has two different thicknesses
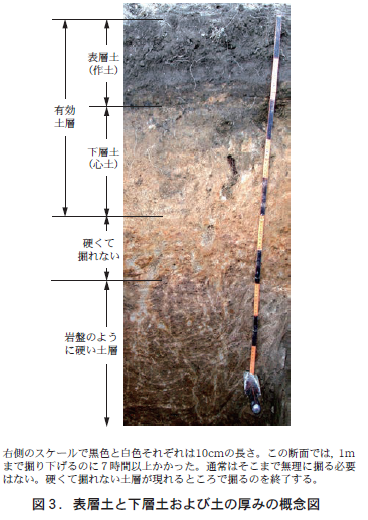
If you look at a cross section of soil, you will notice that there is a layer of black soil at the top of the cross section and a layer below that in which the black color has disappeared (Figure 3). This black layer of soil is called the surface soil (or crop soil). The layer of soil below this layer is the subsoil (or heartsoil). For the sake of convenience, we will consider the subsoil to be the area from the boundary with the surface soil to the point where it cannot be dug with a shovel.
There are two types of soil thicknesses that are one of the conditions for good soil for crop production. One is the thickness of the surface soil. The other is the thickness that allows roots to grow comfortably in the soil. This range of thickness is called the effective soil layer (Figure 3).
To be a good soil for crop production, the thickness of the surface soil should be about 20 to 30 cm, and the effective soil layer should be at least 50 cm thick. For example, in the cross section of soil shown in Figure 3, the thickness of the surface soil is about 20 cm. The thickness of the subsoil from the boundary to the point where it is too hard to dig is about 30 cm, so when combined with the thickness of the surface soil, the effective soil layer is about 50 cm. Therefore, it can be judged that the soil in Figure 3 just barely meets the two thickness requirements for good soil.
3. surface soil thickness is closely related to tillage operations
表層土は土の断面の最上部にあって黒色を呈している。耕地では栽培が終わった後の収穫残渣や,土の中に残された作物の根,さらには堆肥なども含めて様々な有機物が土の表面に添加される。それらはプラウ耕で土にすき込まれ,ロータリ耕で土と混和される。土と混和された有機物は土の中の微生物によって徐々に分解されていく。微生物によって分解されにくい部分が土に残って土の有機物(腐植)になる。土の有機物(腐植)は黒色の複雑な有機化合物であるため,それが表層土の色に黒さを与える。
As the added organic matter is repeatedly incorporated and mixed deeper into the soil, the thickness of the surface soil gradually increases. Thus, the surface soil is affected by human influence. Therefore, if the thickness of the surface soil does not reach 20 cm, which is the lower limit of the target value, it can be increased by actively feeding organic matter such as compost.
This topsoil is sometimes referred to as crop soil in agricultural terms. However, the thickness of the two is not always the same. The crop layer is the layer of soil within the area where the soil is plowed inverted by the plow. The depth at which the soil is plowed is the thickness of the crop layer.
The surface soil provides a place for crop roots to establish and absorb nutrients. In other words, this is the soil layer that has the greatest influence on crop growth. Human activities on the soil for crop production are also basically directed to the surface soil. The management of the surface soil is an important factor in determining the crop productivity of a field.
4. soil thickness that allows roots to grow
根が伸びていくことができる土の厚み,すなわち,有効土層の厚みを営農作業で増やすというのは事実上不可能である。表層土に対してなら,すでに述べたように人為的な改善が可能である。しかし,その下の下層土は土木工事でもしない限り,人の操作で厚みを改変することはできない。有効土層を決める大きな要因は,根が通過できない石ころ(レキ=礫)の層や岩盤,さらにち密で硬い土層などである。こうした層は土木工事で除去しないかぎり移動していかない。このため,私たちが営農作業などで改善できるものではない。
Figure 2 shows an example where a large amount of leki is present at about 50 cm from the soil surface, which limits the thickness of the effective soil layer. It is possible to use a machine to determine whether or not there is any rubble in the subsoil layer. However, such exploration is not common. Also, the presence of the hard, difficult-to-excavate soil layer shown in Figure 3 in the soil can basically only be determined by digging.
The effective soil layer is the soil layer within which the crop roots can spread. If the effective soil layer is too thin, not only will it not be able to adequately support the crop, but it will also limit the area where nutrient water can be absorbed. If the effective soil layer is restricted to a thickness of less than 50 cm, crop growth is inhibited. However, it is quite difficult to artificially increase its thickness.
−第1章− 作物にとってよい土であるための条件
第3回 よい土の条件 物理的性質−その2
土の硬さはどのようにしてきまるのか
令和3 (2021) 年 7月号 (第732号)
Last month, we discussed soil thickness in terms of "soil thickness and hardness," one of the four conditions for good soil for crop production, which is related to the physical properties of soil. This month, we will focus on soil hardness.
1. what determines soil hardness
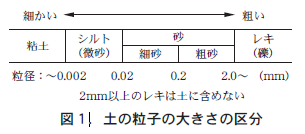
土にはスコップで掘ることができないくらい硬い土もあれば,楽々と掘ることができる土もある。いったい,土の硬さは何によって決まるのか。基本的には「土の粒」の大きさ(粒径という)が土の硬さを決めている。土の粒?と不思議に思う人がいるかもしれない。しかし,砂と粘土を思い出してほしい。砂はザラザラして目で見て一粒一粒を確認できる。しかし粘土の粒は,目で見て大きさを確認できず,かたまりでしか見られない。
When the organic matter contained in the soil is completely removed and only the soil particles are left, the soil is composed of three types of particles: sand (classified into two types: coarse sand and fine sand), clay (clay here does not refer to clay used for clay works but to the very fine soil particles shown in Figure 1), and silt (fine sand), which is intermediate in size between the two. The problem is how much proportion these three types of particles have. The question is what proportion of these three types of particles make up the soil.
2. the finer the soil grains, the harder the soil.
If a glass bead is packed in a certain container, the smaller the bead is, the more space it has. If the glass beads are large, they cannot be packed without gaps, and many spaces are created. The term "denseness" is used to describe how many particles are packed in a certain volume. The degree of density becomes larger when it is packed with a small glass ball than when it is packed with a large glass ball. Clay has the finest grains, so clayey soil has a high degree of density. On the other hand, sand has coarse grains, so sandy soil has a small degree of density. Therefore, clayey soil is tightly packed into a certain volume and becomes hard soil. Sandy soil with coarse particles does not often produce soil as hard as clayey soil.
3. how is soil grain size determined?
Then, how is the size of soil grains determined? If soil grain size is innately determined, there would be no difference between sticky soil and sandy soil. Soil grain size is related to the way soil is formed.
The raw material of soil is basically rock (parent rock) (with the exception of black earth, which is derived from volcanic ash). The rock is broken into small pieces by weathering. The finely crushed rock is called the parent material of soil. The size of the particles that make up the soil is determined by the quality of the rocks used as raw material and the degree of weathering. If the soil has been exposed to weathering for a long time or if the rock is brittle and susceptible to weathering, the soil will have more fine particles, such as clay and silt. This results in fine-grained soil. The opposite is sandy (coarse-grained) soil. The intermediate state between the two is medium-grained soil.
4. very long time is needed to change the hardness of the soil
The hardness of soil is due to the finer and denser particles of soil. The soil particles contain the time it took for the rocks to break up and for the soil to be formed by the action of living organisms. Thus, it takes a daunting, very long-term effort to essentially soften the hardness of the soil.
For example, by feeding coarse organic matter, such as compost, to the soil over many generations, a cushion of organic matter is created in the soil, which gradually softens the soil. In addition, one might think that mixing in sand or other materials would be a good idea. Of course, this is theoretically possible. It may be possible in a small area such as a home garden. However, in a large field, sand must be nearby in large quantities. Sand from the coast is not suitable because of its salinity. It must be river sand. Considering these factors, bringing sand into a fine-grained soil is a picture-perfect and unrealistic idea. It is impossible to change clayey soil into sandy soil.
5. soil hardness is not determined by soil particles alone
So far, for the sake of simplicity, I have explained soil hardness only in terms of soil particles. However, soil hardness is not that simple. For example, soil hardness varies depending on the moisture content in the same soil. This can be seen from the fact that clay soil becomes hard and hard when it is dried. If such soil is gradually moistened with water, it becomes softer and softer, and finally liquefies into a sludge, which is outside the concept of hardness.
The nature of soil hardness is very complex, even when we speak of soil hardness in a nutshell. Here we are simplifying the story.
6. hardness is not the only thing that affects root elongation
Last month and this month we discussed the issue of soil thickness and hardness. This is because we wanted to consider the thickness of the effective soil layer in which roots can grow. However, whether the roots can grow or not is not only determined by the hardness of the soil. There are three factors that inhibit the growth of crop roots. (1) mechanical resistance derived from soil hardness, (2) soil aeration, which indicates whether sufficient air is being pumped into the soil so that root respiration is not adversely affected, and (3) soil moisture conditions. These factors are not independent of each other, but are interrelated, making them even more complex.
7. Hardness is not an inhibiting factor in general soil
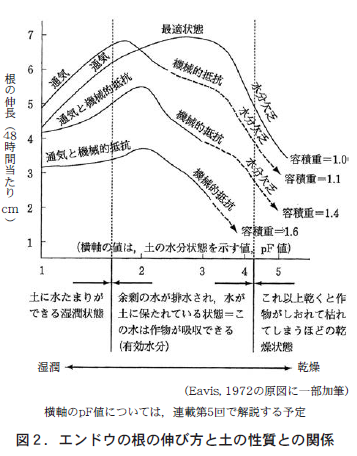
Figure 2 shows the results of an experiment examining the relationship between the above three factors and root elongation. The volumetric weight of 1.0 in Figure 2 indicates that 1 cm3 contains 1 g of dry soil, which is a very normal value for ordinary soil. The increase in volumetric weight can be thought of as the soil particles becoming finer and more cohesive.
Therefore, according to Figure 2, when the soil is wet enough to form puddles, the gaps in the soil are filled with water and the soil lacks oxygen (poor aeration). Therefore, regardless of the volumetric weight of the soil, peas cannot fully extend their roots due to lack of oxygen.
When there is no puddle and the soil contains some water, mechanical resistance, i.e., soil stiffness, will now inhibit root growth. However, this is the case for slightly fine-grained (clayey) soil with a volume weight of more than 1.1. Ordinary soil with a volume weight of up to 1.0 has water and air in the spaces between the soil, so it is in optimum condition for root growth.
When the soil becomes dry, mechanical resistance due to hardness inhibits root elongation in soils with large volumetric weights (fine-grained and clayey). However, in a typical soil with a volume weight of 1.0, mechanical resistance due to hardness does not inhibit root elongation. And, of course, in a soil so dry that the crop wilts, lack of water inhibits root elongation regardless of the volume weight.
In other words, it is concluded that soil hardness is a problem in fine-grained soil (clayey soil), and that in general soil hardness itself should not be considered to be an impediment to root growth. For soils with low volumetric weight and light weight, such as black granite soil (soil derived from volcanic ash), soil hardness is hardly a problem. In fact, because of its lightness, black soil is susceptible to wind erosion (soil being blown away by the wind).
−第1章− 作物にとってよい土であるための条件
第4回 よい土の条件 物理的性質−その3
断面でわかる排水の良否
令和3 (2021) 年 8/9月合併号 (第733号)
In this issue, we will first consider how to judge whether the soil drains well or not, based on the second of the four conditions for good soil for crop production, which relates to the physical properties of the soil, namely, that it retains moisture reasonably well and drains well.
1. observe the cross section of the soil closely
For example, if you have experienced a puddle of water in a field after a heavy rainfall, and the water remains there indefinitely, you should be able to feel that the soil has poor drainage properties. However, if you want to know more about how bad the drainage is, dig a hole in the soil to make a cross-section of the soil and observe it, as described in the second article of this series (June issue). You can determine whether the soil drains well or not by looking at the cross section of the soil as described below.
There is no special pattern in the cross-section of normal soil (Figure 1-a). The soil has a blackish topsoil followed by a brownish-brown subsoil with a fading black color. Soil with such a cross-section is considered to have good drainage.
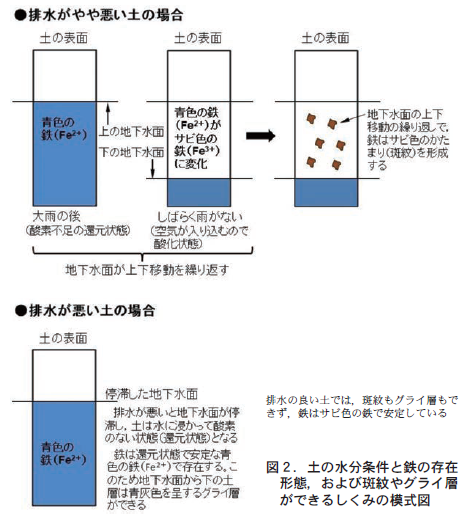
However, you may find an iron rust (brownish-brown) pattern (this is called a mottled pattern) or, in some cases, a bluish-gray layer of soil (this is called a gully layer), as shown in Figure 1-b) on the cross section (Figure 1-c). When mottling is present, drainage is judged to be somewhat poor, and a gully layer indicates that drainage is so poor that groundwater stagnates.
Why is it possible to determine good drainage from mottled or blue-gray soil? It is due to the nature of iron, which is contained in large amounts in the soil (Figure 2).

2. mottled cross section = soil with slightly poor drainage
畑の土は,空気が土のすき間にはいり込むので酸素が存在している。このような状態を酸化的状態という。酸化的状態にあると,土の鉄は水に溶けないサビ色の鉄として存在している。ところが土の排水がやや悪いと,大雨が降ったときに雨水が土の中で滞留し,結果的に地下水位が上がってくる。すると,一時的に土のすき間が水で満たされて酸素不足の状態になる。これを還元的状態という。酸化的な時の鉄はサビ色で水に溶けない状態なのだが,還元的な状態になると,鉄は青灰色の鉄に変化して水に溶け出す。しかし,その後再び乾燥が続くと,排水がまったく悪いわけではないので,少しずつ土の中の水が排水されていき,地下水位が下がって土のすき間に空気がもどってきて酸化的になる。そうすると,鉄は再び水に溶けないサビ色の鉄にかわる。
As the groundwater rises and falls with each rainfall, and the soil repeatedly goes through oxidizing and reducing states, the iron that dissolves during the reducing state collects and clumps together. This is the rusty iron mottling. Since mottling is not produced unless the groundwater level rises and falls, the presence of mottling indicates that the groundwater rises and falls because the soil drainage is somewhat poor.
3. blue-gray layer in cross section = poorly drained soil
On the other hand, if drainage is really poor, the groundwater table is relatively high because water is stagnant in the soil. Thus, the soil immersed in groundwater remains in a reduced state because the crevices are filled with water. As a result, iron continues to exist as blue-gray iron that is soluble in water, resulting in the formation of a blue-gray gley layer. Therefore, the presence of a gley layer means that groundwater is stagnant up to that point and that the soil drains very poorly.
ちなみに,この青灰色のグライ層の土を取り出し空気に触れさせると,空気中の酸素によって徐々に酸化され,サビ色(茶褐色)の水に溶けない鉄の形態に変化していく。土の色に与える鉄の面白い性質である。
4. method of improving soil with poor drainage
Now that we know whether drainage is good or bad, how can we improve soil with poor drainage? In a small-scale field such as a vegetable garden, there are methods such as making ditches to allow stagnant water on the ground surface to flow out, or raising the height of the cultivation area (ridges).
However, in a large area such as a farmer's field, civil engineering work is required to dig a drainage ditch (this ditch is called a culvert) or to bury a drainage pipe under the field (this is called a culvert) and connect it to a drainage ditch (Figure 3).

−第1章− 作物にとってよい土であるための条件
第5回 よい土の条件 物理的性質−その4
適度に水を保持し排水もよい土とは
令和3 (2021) 年 10月号 (第734号)
The second of the four conditions for good soil, which concerns the physical properties of the soil, is that the soil should "retain a moderate amount of moisture and have moderate drainage. In the previous article, I described how to determine whether the soil drains well or not from the cross-section of the soil. This time, we will consider how soil retains water, the fact that some water in the soil can be used by crops and some cannot, and what kind of soil retains water adequately and drains well.
1. capillary tension is the key to water retention and drainage
See Figure 1. Two types of glass capillaries of different thicknesses are placed in water dyed with blue ink (hereinafter referred to as "ink"). In both glass capillaries, the surface of the water rises slightly. This is called capillary action, and the force that pulls up the water is capillary tension. The reason why the ink is raised higher in the thinner glass capillary is because the capillary tension is stronger than in the thicker glass capillary.
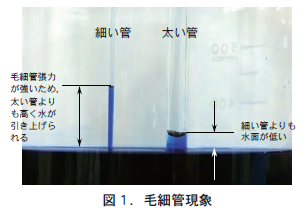
When this glass capillary was lifted above the surface of the ink, the ink in the thin glass capillary remained in the capillary, while the ink in the thick capillary fell to the floor (Figure 2). The capillary tension in the thin glass capillary is stronger than that of gravity, and thus water is retained in the capillary. However, the capillary tension in the thick glass capillary is weaker than the downward pull of gravity, and as a result, the ink fell out of the capillary (drainage).
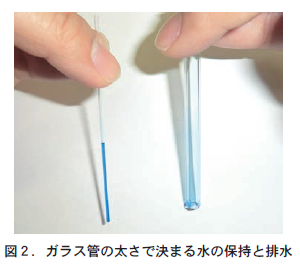
Let us consider this in terms of the gaps in the soil. The space between particles in soil is composed of small, thin gaps and large, thick gaps. Sandy soil with coarse grains (coarse-grained soil) has fewer small gaps and more large gaps, so the capillary tension in the gaps is weak and water tends to drain out. This results in poor water retention, which can lead to drought damage to crops. On the other hand, fine-grained and cohesive soil (fine-grained soil) has many small gaps and few large gaps, so the capillary tension in the gaps is strong. Therefore, water is retained in the narrow gaps, resulting in poor drainage.
2. water that can and cannot be used by crops
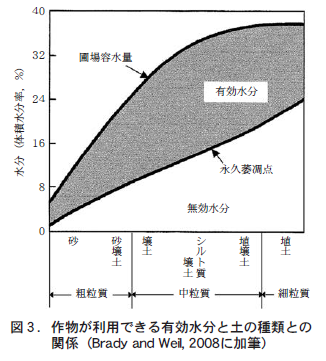
Imagine that there has been a very heavy rainfall and that all the crevices in the soil have been filled with water. There is no space for air to enter the soil, and there are only soil particles and water. The amount of water in the soil at this time is called the "maximum water capacity. However, about 24 hours after the rain stops, the water that was held in the thick crevices by a force weaker than gravity is pulled down by gravity and drains away. Air enters the gap where the water has been drained away. The amount of water in the soil at this time is called the "field water capacity. Of course, the drained water cannot be absorbed and used by the crop.
この状態からしばらく雨がないと,土は乾燥していく。乾燥が進むと,土に水がまったくなくなったわけではないのに作物はしおれていく。この時,水を与えてやるとしおれが回復することはよく経験する。しかし,水が与えられることなく乾燥がさらに続くと,作物はしおれて枯死してしまう。ただし,そんな時でも土の中の水が完全に消えてなくなったわけではない。この時の土の水分量を「永久萎凋(いちょう)点」という(萎凋とはしおれるという意味) 。この時の土の水は非常に細かいすき間や粘土(粒径が0.002 mmより細かい土の粒子)などに,作物の根の吸水力以上の力で保持されている。そのため,土から水が完全になくなっていないのに,作物は水を吸収できずしおれて枯れてしまう。
Ultimately, the amount of moisture that is available to the crop in the soil is the amount of moisture at the permanent wilting point minus the amount of moisture at the field capacity, which is the state after drainage is completed. This moisture available to the crop is called effective moisture. The water remaining in the soil at the permanent wilting point is called invalid moisture because it is not available for crops.
3. soil type and effective moisture content
Soil grain size and effective moisture content are very closely related (Figure 3). Coarse-grained soil has many large, thick crevices and good drainage, resulting in low water content at field capacity (Figure 3). As the soil grains become finer, the amount of water held in the soil increases due to the increase in the number of small crevices, and the amount of water in the field water capacity also increases. However, when the soil grains become finer to a certain degree, the large gaps related to drainage do not change, so the field water content does not change significantly even if the soil grains become finer, and the field water content reaches a ceiling (Figure 3).
一方,作物がしおれて枯れるほどになった時の土の水分量,すなわち永久萎凋点の水分量は,土の粒が細かくなればなるほど直線的に増えていく(図3) 。これは,土の粒が細かくなるほど粘土分や非常に細かいすき間が多くなって,そこに強力に保持される水分量が多くなるためである。したがって,圃場容水量と永久萎凋点の差である有効水分量は,中粒質くらいの土で最大になる(図3)。
4. soil with reasonably good drainage and water retention and how to determine this
よい土である4つの条件の一つ,「適度に排水がよく,なおかつ適度に水分を保つ」という矛盾する条件をうまく満たす土というのは,極端な粗粒でも細粒でもない中粒質の土である。中粒質の土は排水用の大きなすき間と,保水用の小さいが細かすぎないすき間の両方をうまく持っている。このほどよいすき間の構成割合が排水を良好にし,有効水分量も多く保持する土をつくりだす。
To determine if the soil is medium-grained, knead the moist soil with your thumb and forefinger and stretch it into a thread. The soil can be considered medium-grained if it can be stretched to the thickness and length of a matchstick, but not beyond that. If the soil is not matchstick-like, but rather difficult to form threads, it is considered coarse-grained soil.
5. the size of soil particles cannot be easily changed
We know that medium-grained soils are reasonably good for drainage and water retention. However, the size of the soil particles is determined by the degree of weathering of the rocks from which the soil is made, which requires time enough to tell the geologic age of the soil. Turning coarse- or fine-grained soil into medium-grained soil is not something that can be done overnight. Creating gaps in the soil for water retention and drainage requires a generational effort to continue applying organic matter such as compost.
−第1章− 作物にとってよい土であるための条件
第6回 よい土の条件 化学的性質−その1
土の酸性度(pH)
令和3 (2021) 年 11月号 (第735号)
I believe that good soil for crop production is soil that meets the four conditions that I presented in the first article of this series (May issue). Of these four conditions, I have discussed two that relate to the physical properties of the soil. From this month, I would like to move on to the two conditions related to the chemical properties of soil. This month, I will discuss one of them, acidity (pH).
1. what is pH?
First, let's talk about the term pH. pH indicates the strength of acidity or alkalinity. pH ranges from 0 to 14, with neutral being pH 7. A pH value less than 7 is acidic, while a pH value greater than 7 is alkaline. Originally, pH was calculated from the concentration of hydrogen ions dissolved in water (strictly speaking, the degree of activity). The higher the hydrogen ion concentration, the more acidic the water is, and the lower the pH value. Conversely, the lower the hydrogen ion concentration, the greater the pH value, and the less acidic (more alkaline) the water. This is a little different from common sense, so it is easy to get confused.
For example, the difference between pH 5 and 6 is 1. The difference between pH 4 and 6 is only 2, but the hydrogen ion concentration is 100 times higher at pH 4.
2. pH conditions suitable for crop production
In Japan, the optimum pH condition for good soil for crop production is in the range of 5.5 to 6.5, which is slightly on the acidic side. Of course, even within this range, the optimum pH varies slightly depending on the crop. For example, spinach, lettuce, chrysanthemum, and tomatoes prefer a pH closer to 6.5. On the other hand, balayasho (Japanese radish) prefers a pH closer to 5.5 for healthy growth, and radish and turnip are also tolerant of acidic conditions. Why is it that the optimal pH of soil in Japan is within this range? The reason is that Japanese soil tends to become acidic when left to nature.
3. factors causing acidification of Japanese soil
So why does Japanese soil become acidic when left to nature? Two factors are mainly responsible: (1) rainwater and (2) chemical fertilizers, which are indispensable for crop cultivation. The two main factors are (1) rainwater and (2) chemical fertilizers, which are indispensable for crop cultivation.
(1) Acidification by rainwater
雨水はpH7の中性ではない。空から落ちてくる過程で,大気中の炭酸ガス(二酸化炭素,CO2)を溶かし込み,天然の炭酸水になっている。そのpHは5.6程度である。わが国は世界的に見ても降水量が多く,この酸性の水が土を洗い流すため,土が酸性側に傾きやすい。
However, rainwater is naturally carbonated water when it falls in ideal, clean, unpolluted air. In areas with severe air pollution, various air pollutants are present in the atmosphere, such as sulfur oxides, nitrogen oxides, and chlorides from the oceans. These substances are also dissolved in rainwater and chemically transformed into strongly acidic substances such as sulfuric acid, nitric acid, and hydrochloric acid, respectively. This results in rainfall with a pH lower than the ideal pH of rainwater (carbonated water) of 5.6. This is acid rain (snow and fog also become acidic through the same mechanism as rain). Even ordinary rainwater, when washed away from the soil over a long period of time, causes acidification of the soil. Needless to say, acid rain (snow and fog) accelerates soil acidification.
(2) Acidification by chemical fertilizers
The second soil acidifying factor is chemical fertilizers. Chemical fertilizers are chemically manufactured crop nutrients. While they themselves may provide nutrients to crops, they do not have any specific detrimental effects on crops. However, if chemical fertilizers are not used properly, they can have a variety of negative effects. Acidification is one of them.
However, not all chemical fertilizers acidify the soil to the same degree. Urea, for example, is decomposed by microorganisms in the soil and then converted to ammonia for use by plants. Therefore, it acidifies the soil to a lesser degree than other fertilizers. Ammonium nitrate (ammonium nitrate) and ammonium phosphate (ammonium phosphate) are also members of this class of fertilizers.
4. why soil acidification is bad for crop growth
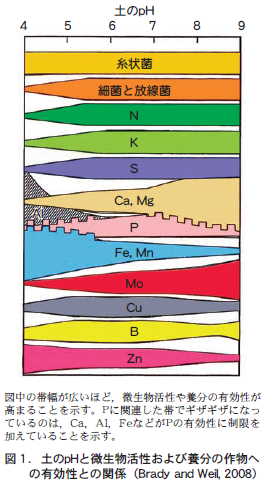
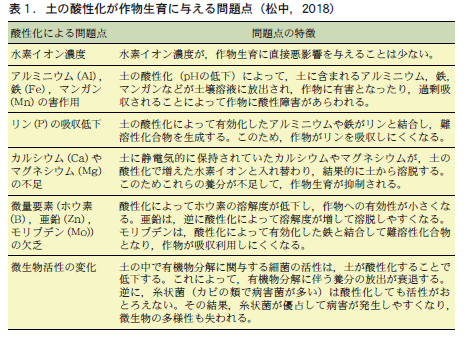
The activity of soil microorganisms and the availability of nutrients to crops are greatly affected by soil pH (Figure 1). Table 1 summarizes the effects of soil acidification (low pH) on crop growth. Basically, soil acidification has a negative effect on crops. The most serious problem is that acidification causes aluminum, iron, manganese, and other elements to dissolve into the water in the soil (soil solution), which in turn damages crops.
アルミニウムは,通常,土の中の粘土鉱物の結晶を構成して存在している。ところが土の酸性化によって増えた水素イオンは粘土鉱物の結晶を破壊し,結晶を構成しているアルミニウムを土壌溶液に溶けている各種の陽イオンと入れ替わることができる形態(交換性アルミニウム)に変化させる。このため,pHが5程度より低下すると,急速に土壌溶液中のアルミニウム濃度が高まる(図1) 。土壌溶液の高アルミニウム濃度は,作物の根の細胞に直接障害を与え,養分吸収を阻害する。
アルミニウムだけでなく,土の中に多く含まれている鉄やマンガンも,酸性化すると急に土壌溶液に溶け出していく(図1) 。鉄やマンガンは,アルミニウムとちがって作物の必須養分である。しかし,土が酸性になって過剰に溶け出すと,作物に過剰吸収害を与え生育に悪影響を与える。
Even more inconvenient is the fact that aluminum and iron are strongly bound to phosphorus, and the combined aluminum and iron phosphates are almost insoluble in water. This makes it difficult to absorb the phosphorus that is fed as fertilizer, and crops are prone to phosphorus deficiency.
5. proper acid amendment of soil as well.
You cannot tell its pH just by looking at the soil. It is practical to have the soil analyzed by soil diagnosis. If the analysis shows that the pH is lower than the optimum pH, the amount of alkaline material (calcium carbonate, etc.) needed to improve the pH to the optimum level is suggested. The acidity can be improved by giving the appropriate amount to the field and thoroughly mixing it with the soil. Conditions related to the chemical properties of the soil are relatively easy to improve, unlike the physical properties.
−第1章− 作物にとってよい土であるための条件
第7回 よい土の条件 化学的性質−その2
酸性障害がでる土とでにくい土
令和3 (2021) 年 12月号 (第736号)
先月は作物生産にとってよい土であるための条件で適正なpH(純水H2Oを使って測定したpH,以下同様)は5.5〜6.5の範囲であると指摘した。適正範囲がやや酸性側にあるのは,わが国では雨が多く土が酸性化しやすい条件にあり,その条件に適した作物が栽培されるからである。また,土が酸性化して作物生育に悪影響を与える要因のうち,とくに悪影響が大きいのはアルミニウム(Al)であることも述べた。
However, there are various kinds of soils that are highly acidic with a pH lower than 5.0 but do not cause serious damage to crops. This month, we will consider why this is the case.
1. soil that allows corn to grow vigorously even in highly acidic conditions
First, look at Figure 1. This is the result of corn cultivation using two types of soil derived from volcanic ash widely distributed in Japan (commonly known as volcanic ash soil, or more correctly, black box soil).
The two soils on the left in Figure 1 are typical Japanese black granite soils called allophenolic black granite soils (hereafter abbreviated as A black granite soil). The name is derived from the presence of clay minerals (such as allophene and imogolite) that do not form a clear crystal structure. On the other hand, the two soils on the right are special black earths distributed in the Tohoku region, Hokkaido, and the Sea of Japan side of Honshu, and are called non-allophene black earths (hereafter abbreviated as N black earths). The main clay mineral in this soil is not allophene, but a clay mineral with a well-defined crystal structure.
The pH of the A black soil was 4.8 and that of the N black soil was 4.5, making them highly acidic soils. We compared the growth of corn plants in these soils with and without acidification with calcium carbonate (calcium carbonate), an alkaline material, after supplying sufficient lime superphosphate as a phosphorus material.
If the soil is highly acidic, as these two A black box soils are, corn growth should deteriorate unless the soil is acidified. However, strangely enough, there was no significant difference in the growth of corn in the left two A black soil, regardless of whether the soil was acidified or not. On the other hand, in the case of the two N black box soils on the right, the growth of corn plants was greatly suppressed without acidification. In other words, there were two types of soils: one in which acid damage appeared in the crop even when the soil was acidified (in the case of the two N black box soils on the right of Fig. 1), and the other in which this did not occur (in the case of the two A black box soils on the left of Fig. 1). Why is this?

2.交換酸度(y1)がちがっていた
通常,土のpHは純水(H2O)を使って測定している。しかし,もう一つの測定方法として,塩化カリウム(KCl)溶液を用いる場合がある。そのときはpH(KCl)と表示する。
The idea of using potassium chloride solution to measure pH came from Gintaro Daikubara, who was the first in the world to research methods of improving soil acidity. He believed that soil acidification adversely affects crops because of aluminum ions dissolved in soil moisture (soil solution), and that the degree of adverse effect is determined by the amount of exchangeable aluminum retained in the soil. As a method to measure this, he devised an ion-exchange method in which the exchangeable aluminum retained in the soil was ion-exchanged with potassium ions in a potassium chloride solution and released into the solution.
土から塩化カリウム溶液に放出されたアルミニウムイオンは,溶液のH2Oと次々に反応して水素イオンをつくりだす。このため,水素イオン濃度が濃くなって酸性度が強まる。この強まった酸性度をアルカリ性の水酸化ナトリウム(苛性ソーダ)溶液で中和し,その中和に必要な水酸化ナトリウムの量(ミリリットル=㎖)で,間接的にアルミニウムイオンを測定しようとした。この㎖値を交換酸度とよび,記号をy1(ワイワン)とした。
図1の実験で用いた二つの黒ボク土のy1は,A黒ボク土の場合4.4であったのに対し,N黒ボク土は28.0と大きくちがっていた。つまり,A黒ボク土は酸性障害の原因物質である交換性アルミニウムがもともと少なく,N黒ボク土はA黒ボク土の7倍近くも多く持っていたことになる。
Therefore, it can be understood that corn, which is relatively tolerant to acidity, did not suffer from growth failure with the amount of exchangeable aluminum as low as that of A black soil, even if the pH was very acidic. On the other hand, if the amount of exchangeable aluminum is high, as in the case of N black soil, the amount of aluminum ions in the soil solution will increase and cause acid damage to the roots if the soil is not acidified.
3.なぜ土によって交換酸度(y1)がちがうのか
The reason that corn could grow without acidification even though the soil is highly acidic is because the A black box soil could not retain much exchangeable aluminum. The question is why.
This is a curious fact, but it is deeply related to the negative and positive electrostatic properties (positive and negative charge) of soil (details will be given later in this series).
A黒ボク土が持つ負荷電や正荷電は,周りの土壌溶液のpHによって大きく変化する(これを変異荷電という) 。この性質をもつ負荷電は,土が酸性に傾くとそれ以上酸性化しないように作用し,プラスの電気を帯びた水素イオンが土壌溶液へ放出されないよう自分の負荷電に静電気的にひきつけて保持した状態を維持する。そうなると,負荷電は水素イオンでふさがれたままなので,空いた負荷電がない。プラスの電気をもつアルミニウムイオンは土の負荷電に引きつけられて交換性アルミニウムとなる。しかし,そのための空いた負荷電がないA黒ボク土では,交換性アルミニウムが安定して保持される場所がなく,その結果として交換酸度(y1)が小さくなる。
一方,N黒ボク土の負荷電はA黒ボク土のような性質がなく,いつでも負荷電として機能している。このためプラスの電気を帯びるアルミニウムイオンが近くにくると,負荷電に保持されていた水素イオンとイオン交換して,アルミニウムイオンが負荷電に引きつけられて保持される。このためN黒ボク土では交換性アルミニウムが安定して存在でき,その量も多くなって交換酸度(y1)が大きくなる。
4.交換酸度(y1)の測定の重要性
日本の土は酸性化しやすい。だから毎年炭カルを与えて酸性化を防がねばならないとしばしば指摘される。このことが強調されるあまりに,土壌診断をすることもなくむやみに炭カルを与えることにつながり,結果的に高pHの土をつくりだしている。酸性改良について,とくに注意すべき土かどうかを見分けるにはy1を測定すればよい。
酸性障害が発生しやすいN黒ボク土は,y1が5以上とされている。作物によって酸性に対する強さ(耐性)がちがうので,酸性障害が発生しやすい土かどうかの判定基準は作物で異なる。このため判定基準を一般化するのは難しい。しかし,その目安の一つとしてy1が5以上かどうかを採用してはどうだろうか。pHの測定と同様,土壌診断の項目にy1を加えることは重要だと思う。
−第1章− 作物にとってよい土であるための条件
第8回 よい土の条件 化学的性質−その3
適度に含まれる作物の養分
令和4 (2022) 年 1月号 (第737号)
Of the four conditions for good soil for crop production, we have discussed three so far. This month, we begin the fourth and final condition. That is, we will consider the chemical properties of the soil, i.e., "the soil must contain an adequate amount of nutrients for crops. The question here is what kind of nutrients are contained in crops and how much are they in moderation.
1. 17 nutrients essential for plants (crops)
We humans obtain the nutrients we need from food, such as carbohydrates, fats, proteins, vitamins, and minerals (inorganic substances). In the same way, plants (crops are included in plants) also need nutrients, which we call nutrients.
とくにアーノンとスタウト(1939)は,次の3つの条件を満たした養分を,植物の生育になくてはならない必須養分と定義することを提案した。現在はそれが受け入れられている。その3つの条件とは,
①その養分がなければ植物は生育し続けることができないこと(必要性) ,
②その養分がなければ固有の欠乏症があらわれ,その症状を正常に回復させる方法はその養分を与えること以外にないこと(非代替性) ,
③その養分が植物の栄養に直接的な役割をはたしていること(直接性)
である。直接的な役割とは,その養分が植物体を構成する成分であるか,あるいは,体内での生理的な反応に直接かかわりをもっているという意味である。
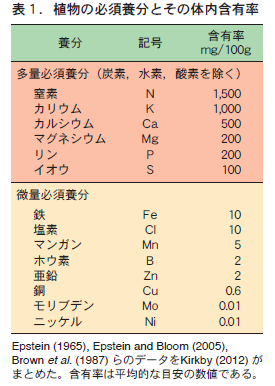
現時点で必須養分は以下の17個である。必須養分のうち植物が比較的多量に要求する養分を多量必須養分といい,炭素,水素,酸素のほかに,要求量の多い順に,窒素,カリウム,カルシウム,マグネシウム,リン,イオウの9つである(表1) 。炭素,水素,酸素は,大気中の二酸化炭素や酸素,さらに,土の中の水(H2O)から吸収できるので,地球上で植物が生育するかぎり不足することはあまりない。したがって,多量必須養分として重要なのは窒素からイオウまでの6種類である。必須養分で残りの8つは,鉄,塩素,マンガン,ホウ素,亜鉛,銅,モリブデン,ニッケルである。これらは比較的わずかな量しか植物に要求されないので,微量必須養分という(表1) 。
2. the controversy and settlement of what nutrients are
In fact, the controversy over what is a nutrient for plants has a long history, dating back to the Greek and Roman periods. The debate began in Germany in the 19th century, settling the debate about nutrients between Thea, who believed that nutrients were organic (carbon-containing substances), and Shupe, who believed that they were inorganic (also known as minerals. The debate between Theja, who claimed that nutrients are organic (substances containing carbon), and Sprenger and Liebig, who claimed that they are inorganic (substances that do not contain carbon).
The theory that organic matter is a nutrient was strongly influenced by the "vital energy theory" that was prevalent throughout society at the time. According to the animate theory, organic matter was produced with the help of a unique life force that existed only in living plants and animals, and that organic matter could not be produced from inorganic matter that did not have life. This argument, however, lost its basis when Weller of Germany synthesized urea, an organic substance, from inorganic substances in 1828. Thus, the controversy was settled on the basis of Sprenger and Liebig's claim that inorganic substances are nutrients. However, the idea that organic substances are nutrients has not disappeared, but has been handed down in the spirit of organic agriculture to this day.
3. moderate nutrient levels and soil diagnostic reference values
By the way, how much of these nutrients must be present in the soil in adequate amounts? Moreover, crop nutrients are not merely sufficient if they are present in the soil, but they serve as nutrients for crops only when they are present in a form that can be absorbed by the crop (this is called the availability form). Therefore, to determine whether nutrients are adequately present in the soil, it is necessary to know how much nutrients are available in the availability form. Unfortunately, this cannot be determined just by looking at the soil. It can only be determined by analysis. In other words, nothing can be known without soil diagnosis.
Soil diagnosis here refers to a series of operations in which the soil is analyzed to determine the pH level and the amount of available nutrients in the soil, to determine whether the analysis results are adequate, and to provide specific measures based on the results. The standard to judge whether the condition of soluble nutrients is adequate or not based on the analysis results is the soil diagnostic standard value. In other words, the appropriate amount of nutrients as a condition for good soil is the amount of nutrients in the soil that falls within the range of the soil diagnostic standard value.
The amount of nutrients required by crops varies greatly from crop to crop. They may also be affected by the climatic conditions of the region where the crop is grown. For this reason, there is no uniform standard value for soil diagnosis that is valid for all crops in the country. Please refer to the information published by the testing and research institutes in your area to find out the specific values of soil diagnosis standards.
4. nutrient supplementation based on soil diagnosis results
If all nutrients are within the range of soil diagnostic standards, the soil is considered to have adequate nutrients. However, this does not mean that compost, chemical fertilizers, or other materials do not need to be applied to the soil. This section introduces the basic concept of nutrient supplementation based on soil diagnosis results from a case study in Hokkaido, Japan.
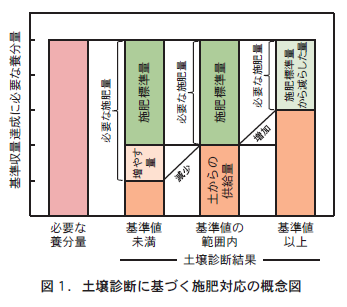
In Hokkaido, the "standard yield" is defined as the yield level that can be achieved through appropriate cultivation management under relatively favorable weather and soil conditions. Under conditions where soil nutrients are within the range of soil diagnostic standard values, the amount of nutrient supply (amount of chemical fertilizer as nutrients = amount of fertilizer applied) required to ensure this standard yield is the "standard fertilizer application amount" (Figure 1). It is based on the concept that the amount of nutrients required for a crop to produce the "standard yield" is secured from both the amount supplied by the soil and the amount supplied by chemical fertilizers and compost.
Therefore, when soil nutrients are below the soil diagnostic standard, the amount of nutrients supplied by the soil is reduced, and the reduced amount must be supplemented with compost or chemical fertilizer in addition to the standard amount of fertilizer to produce the standard yield. Conversely, if soil nutrients are above the soil diagnostic standard, the amount of nutrients supplied by the soil will increase, and the reduced amount of nutrients from the standard amount of fertilizer will be sufficient (Figure 1). As mentioned above, the Hokkaido Fertilizer Guide 2020 provides specific numerical values for each crop as to how much to increase or decrease the standard amount of fertilizer based on the results of soil diagnosis. It may be helpful for your reference.
−第1章− 作物にとってよい土であるための条件
第9回 よい土の条件 化学的性質−その4
土が養分を保持するしくみ
令和4 (2022) 年 2/3月合併号 (第738号)
It is hard to believe that soil is electrostatically charged. However, because of this property of soil, nutrients contained in fertilizers and compost are attracted to and retained in the soil by electrostatic forces. Those who discovered the soil's ability to hold these nutrients (nutrient retention capacity) must have been surprised, too.
1. discovery of nutrient retention capacity of soil
The story goes back to England about 170 years ago. At that time, chemical fertilizers were new to the world and were rarely used. Therefore, the source of nutrients for crops depended exclusively on compost produced from livestock manure. However, the ammonia contained in the manure would volatilize and lose if left unattended. To prevent this, sulfuric acid, a byproduct of coal combustion, was diluted and spread on the compost heap.
However, the sulfuric acid treatment resulted in the production of large amounts of ammonium sulfate. Thompson, a wealthy farmer in Yorkshire, northern England, wondered if the sulfuric acid treatment would actually reduce the fertilizing effect of the compost because rainwater would dissolve the ammonium sulfate and run off into the ground. Thompson asked Spence, a pharmacist with a background in chemistry, to test the veracity of his suspicions.
スペンスは農場の土に硫酸アンモニウムを与えてよく混ぜ,それをガラス管に詰めて上から蒸留水(H2O)を流し,下から出てきた浸透水の成分を分析した。すると,添加したはずのアンモニウムが浸透水から消え,代わりにカルシウムが硫酸カルシウムとなって現れた。このスペンスの実験結果から,トンプソンは土がアンモニウムをひきつけて保持したと考え,世界で最初にこの事実を論文発表した。1850年のことである。
Around the same time, Huxtable of Dorset, southern England, also recognized the ability of soil to purify the color and odor-causing substances in manure mixtures.
After hearing the results of these experiments at a meeting of the Royal Agricultural Society of England, Ouray followed up their experiments himself and reaffirmed that the results were true. Later, after five years of extensive experimentation, he found that the clay of the soil had the ability to attract substances. The paper by Ouray describing these findings appeared in the same issue of the same journal in which Thompson's paper appeared, but on a page behind Thompson's.
All of them, who were interested in similar phenomena in the same period, concluded that this property of the soil would play a major role in actual agriculture. Among them, Ouray even believed that this soil property was due to ion exchange occurring in the soil. However, it was not until some 40 years after his death that this idea was accepted by the public.
2. the carrier of the soil's nutrient retention capacity
The results of Wei's experiments led to research on soil organic matter and clay minerals and their ability to retain nutrients, which were found to have electrically charged properties. It was found that soils can be negatively electrically charged (negative charge) or positively electrically charged (positive charge) in some cases.
(1) Bearer of soil load (negative electricity)
土の負荷電の担い手は,①粘土鉱物の構造変化による荷電,②粘土鉱物の端末にできる荷電,③有機物(腐植)の端末にできる荷電の3つである。
粘土鉱物とは土の原料である岩石(一次鉱物)が物理的,あるいは化学的な風化作用をうけて変成し,元の岩石とはちがう鉱物(二次鉱物)となったものである。基本となる構造はケイ素もしくはアルミニウムが主体となり,それに酸素,水素などが規則性をもって結合した1枚の面状になっている。ここではケイ素主体のシート,アルミニウム主体のシートということにする。
負荷電の担い手の①は,この粘土鉱物の結晶,たとえばケイ素主体のシートで,ケイ素(プラスの電気を帯びる手(正荷電)の数が4つ)が,原子の大きさがほぼ同じのアルミニウム(正荷電の数が3つ)と入れ替わる(同型置換という)ことによってできる(図1) 。正荷電4つのケイ素は,酸素の負荷電4つとつりあっていた。ところが,正荷電3つのアルミニウムと入れ替わると,酸素の負荷電1つに余剰がでる。この余剰の負荷電は,周りの条件にかかわらず常に負荷電として機能する安定した荷電で,永久荷電という。
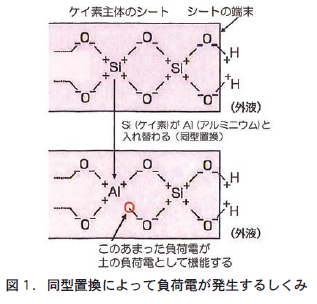
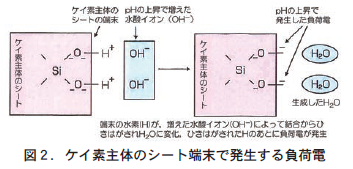
負荷電の担い手の②と③は,いずれも荷電の周りのpHが上がるとともに発生する。pHが上がるというのは,水素イオン(H⁺)が減少して水酸イオン(OH⁻)が増えることを意味している。②の場合も③の場合も,粘土鉱物のシートや有機物の端末にある水酸基(−OH)やカルボキシル基(−COOH)の端にある水素(H)が,増えた水酸イオンにひき寄せられ,結合している酸素(O)からひきはがされてH2Oをつくることで負荷電に余剰が発生し,土の負荷電として機能する(図2) 。つまり,この負荷電はpHの影響を受けて変化する不安定な荷電で,変異荷電という。
(2) Bearer of positive charge (positive electricity)
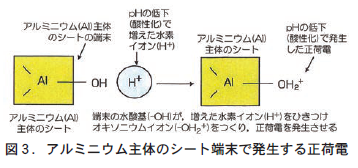
土の正荷電の担い手は,①アルミニウム主体のシートの端末にできる荷電,②有機物の端末にできる荷電,③風化が進んだ土の粘土鉱物(鉄やアルミニウムの酸化物)にできる荷電の3つである。
これら3つの荷電はいずれも周りのpHが下がるとともに発生する変異荷電である。pHが下がるということは水素イオン(H⁺)が増えることを意味している。正荷電が発生する基本的なしくみは,①②③のいずれも同じで,それぞれの端末にある水酸基(−OH)やカルボキシル基(−COOH)などがpH低下で増えた水素イオン(H⁺)をひき寄せてオキソニウムイオン(−OH2⁺)をつくり,これが正荷電として機能する(図3) 。
3. permanent charge and exchangeable aluminum
先に指摘した永久荷電の負荷電はpHの影響を受けない。このため,酸性化してpHが低下しても負荷電として機能しているので,酸性条件で現れるアルミニウムイオン(Al3⁺)を交換性陽イオンとして安定して保持できる。本連載第7回で紹介した非アロフェン質黒ボク土はこのタイプの負荷電を持っており,作物の酸性障害の原因となる交換性アルミニウムを多く保持できた。トウモロコシに酸性障害が発生したのはこのためである。
On the other hand, allophenic black box soils are predominantly mutagenically charged from organic matter and become positively charged upon acidification. Exchangeable aluminum, which has the same positive charge, repels the positive charge of the soil and cannot exist stably. Therefore, it can be understood that the allophenolic black box soil did not cause acid damage to the corn. This is a very interesting phenomenon.
−第1章− 作物にとってよい土であるための条件
第10回 よい土の条件のまとめ
−どんな土でも必ずよくなる−
令和4 (2022) 年 4月号 (第739号)
It has been a year since I began writing this series of articles in last May's issue. Over the past nine articles, we have discussed four conditions that must be met for soil to be good for crops. These four conditions and specific target values were described in the first article. From Part 2 to Part 9, each of these conditions and their target values were explained. This issue is a summary of these articles. I would like to review the relationship between the concept of good soil and soil cultivation.
1. what is soil preparation in the first place?
When farmers, as well as readers of this magazine, and others involved in agriculture discuss crop production and talk about soil, the word "soil preparation" always comes up. The final response is something like, "Soil preparation means the application of organic matter such as compost. The conclusion is that no matter what kind of soil or what kind of crops are grown, the first step in "soil preparation" is to apply compost, and if that "soil preparation" is carried out, it will lead unconditionally to good results.
このような「土づくり」に対する固定概念に対して,なんともいえない違和感がある。そんな単純な話であるならば,この世の中で作物生産の劣る土は,そのうちなくなってしまうと思うからだ。要するに堆肥を施用すればよくなるのだから。
I consider "soil building" to be a practical activity to improve the soil of a target field to a soil that is good for crop production. In order to do so, it is necessary to clarify what factors in the soil of the field are inhibiting the growth of crops and to what extent. In other words, it is necessary to clarify which of the "four conditions for good soil" described in this series of articles is the greatest inhibitory factor to the growth of crops. Then, the practice of "soil cultivation" is the procedure of implementing measures to improve the conditions that have become the growth-inhibiting factors.
2. does good soil guarantee a high yield?
In reality, however, there is more at stake. Crop growth and yield are not determined solely by whether the soil on the farm is good for crop production.
Suppose there is a potato field with the best soil in Japan that satisfies all the "four conditions for good soil" through diligent improvement. However, no matter how good the soil is, if the temperature does not rise in summer, for example, the productivity (yield) of the potato field will be drastically reduced due to cold damage. Even if the weather is good, if the fertilizer is applied incorrectly, the potatoes will not produce well. Experienced farmers will be able to produce much higher yields than I, an amateur, can.
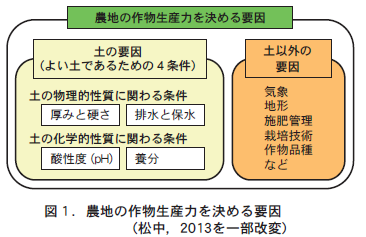
Crop productivity of soil and crop productivity of farmland have different dimensions. No matter how good the soil is for crop cultivation, crop productivity of farmland depends on many factors other than soil, such as weather, topography, site environment, fertilizer application and cultivation techniques, as well as the type of crop grown and the variety of the same crop (Figure 1).
3. soil is one of the factors that determine the crop productivity of farmland
In order for soil to have high crop productivity, it must satisfy all of the "four conditions for good soil," as described in this series of articles. However, it cannot be said that farmland with "good soil" that satisfies these four conditions will always produce a high crop yield. This is because "good soil" is only one of many factors that determine the productivity of farmland, as shown in Figure 1.
The more we value the soil, the more we feel that the soil always determines crop production. As a result, it is often said that "soil cultivation" is the most important factor in "improving crop productivity. Of course, there are many such cases. However, if we assume that soil is the only factor that determines crop production on farmland, we will lose sight of the real impediments to crop production. We must not forget that many factors are interrelated in determining the crop productivity of farmland.
The important thing is to be able to consider from a broad perspective what factors are responsible for the poor growth of crops grown on the farm. It would be a pity for the soil to blame the soil for all the causes of various phenomena, such as poor growth of crops. That is an overestimation of the soil. Before we assume that the soil is the cause, it is important to consider the factors that contribute to poor growth, and to accurately gather the factors that hinder growth.
4.「木を植えた人」の話
−どんな土でも必ずよくなる
I will never forget the words of my former teacher when I was a student: "Any soil can be made better. This is because I believe that even soil with many shortcomings will one day improve if we find out which of the "four conditions for good soil" are the factors that inhibit the growth of crops in that soil, and then do our best to improve those factors.
とりわけ土の物理的性質にかかわる条件の本質的な改良は,「堆肥をやればよくなる」というような一朝一夕でできるものではない。その改善対策を,親−子−孫と世代を超えて継続しなければ,おそらく実現できないだろう。問題は,それまで,あきらめることなく倦まずたゆまず,改善の努力を持続できるかどうかである。
There is a French literary figure named Jean Giono. The soil in the Provence region of southern France, where he was born, is often so barren that the surface soil is thin and marble (limestone) is readily visible (Figure 2). However, he never left his birthplace throughout his life, loving the land and writing his literary works there. One of his best-known works is "The Man Who Planted Trees," which is well known. It is the story of Elzéard Bouffier, who planted trees on the barren soil of Provence, restored forests and rivers, and even restored the moisture to people's hearts.
Selflessly and without asking for anything in return, day in and day out, he drilled holes in the barren, marble-covered soil and planted acorns in them. This act transformed the barren land into a land fit for people to lead healthy lives, both physically and mentally. I believe that with a sustained selfless practice like Bouffier's, any harsh, poor soil can be transformed into soil that can produce crops and in which people can live richly in mind and body.
What is necessary is to clarify what factors of the soil need to be improved and how, and then to continue with the improvement measures. We hope that you will utilize the "Four Conditions for Good Soil" to find these factors. To change bad soil into good soil for crops requires work like bouffier. Simply applying compost may miss the point.

−第2章− 堆肥と化学肥料
第11回 堆肥は養分移転資材として登場した
−養分の補給方法を考える−
令和4 (2022) 年 5月号 (第740号)
Starting with this issue, "No More Soil" will be renewed. In the past 10 articles, we have discussed the four conditions for good soil for crops. In the next 10 articles, we will discuss compost, chemical fertilizers, organic farming, and the role of soil. This time, we will look back at the history of compost, which was invented and used to replenish farmland with nutrients in the days before chemical fertilizers were available.
1. nutrient replenishment is necessary to maintain soil fertility
It is believed that humans began farming about 10,000 years ago. Since then, when crops are grown and harvested on farmland, the nutrients in the soil are absorbed by the crops and taken out of the farmland with the harvest. If nutrients are not replenished, the crop nutrients in the soil will be depleted and the crop cannot be grown. Therefore, replenishment of nutrients was essential for the sustainable cultivation of crops on farmland.
How exactly to supply nutrients was the main concern. The only way to recover nutrients from the soil was to collect materials around us, such as leaf litter from forests, mud from rivers and lakes, fallen leaves, fallen branches, underbrush from forests, wild grasses, grass and tree ashes, seaweed, and manure from people and livestock. This nutrient transfer process was very labor intensive and time consuming.
2. compost conceived as a nutrient transfer material
The most powerful material ever conceived for recovering crop nutrients from the soil and transferring the recovered nutrients to another location was compost. There were two ways to recover nutrients from the soil. One was the conventional method of cutting plants that grow outside the farmland (wild grasses, weeds, etc.), collecting fallen leaves and branches, and undergrowth from the mountains and forests, and letting them accumulate and decompose to make compost. The other method is to feed livestock with plants that can be used as fodder (wild grasses and pasture grasses), and collect the nutrients absorbed by the plants in the soil in the form of livestock manure for composting.
European farmers, in particular, realized that the use of livestock was much more labor-saving than collecting materials from outside the farmland. Therefore, they actively promoted nutrient recovery and composting through the use of livestock and developed a crop rotation system. The culmination of these efforts was the four-year crop rotation known as the Norfolk farming method.
3. intensive crop rotation before the advent of chemical fertilizers
ヨーロッパでの輪作の初期は単純に農地を二分し,一方は作物栽培に用い,他方は作物栽培を休む(休閑という)ことで,土の中の養分の回復を自然にまかせるという二圃式輪作であった。その後,三圃式,穀草式と発展し,ノーフォーク農法(輪栽式)にたどり着いた(図1) 。それまでの輪作でも,共有地や永久放牧地で放牧される家畜のふん尿を利用した。しかし,放牧地や草地で家畜を飼養すると,そこで排泄される家畜ふん尿の回収率が低下するという難点があった。さらに,秋から冬にかけての期間は野草や牧草の生育が衰えるため,家畜を越冬させるのに十分な飼料が確保できなかった。
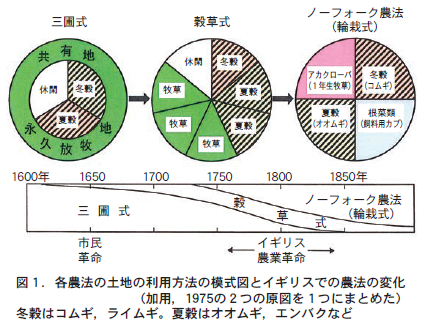
The Norfolk farming method expanded farmland by eliminating all communal grazing land and fallow, and ensured fodder production by incorporating two crops for livestock fodder (fodder turnips and red clover) into a four-year crop rotation. For sowing fodder turnips, a strip sowing method was introduced using a strip sowing machine developed by agricultural experimenter Tal. This made it possible to use a weed control tiller and stabilize the production of fodder turnips. Red clover is expected to fix nitrogen by rhizobium bacteria that live in symbiosis with the roots, and was effective in increasing the nitrogen fertility of the soil. This has led to increased fodder production, and has made it possible to keep a large number of livestock and to keep them in sheds even during the winter. Keeping livestock in sheds increased the manure collection rate and dramatically increased the amount of manure produced. This has led to an increase in the amount of manure applied to grain-producing fields, which in turn has helped to maintain high soil nutrient fertility (Figure 2). Thus, an agricultural method based on nutrient cycling using livestock manure was established, and a super-intensive crop rotation was realized at that time.
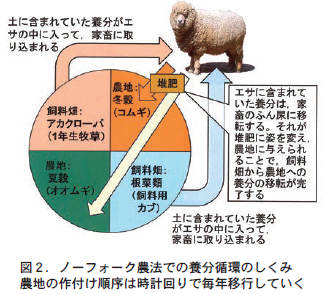
There is a saying in the Flemish region of mainland Europe across from Norfolk, England (present-day southern Netherlands to western Belgium and northern France): "Without feed there is no livestock, without livestock there is no fertilizer, and without fertilizer there is no harvest. This saying eloquently describes the major role of manure as a nutrient transfer material for maintaining crop production in European crop rotation.
The Norfolk farming system transformed wheat production. Although Norfolk is a small region with only 41 TP3T of the area of England (one of the four constituent kingdoms of the United Kingdom), the introduction of the Norfolk farming method covered 901 TP3T of the entire England at that time in terms of wheat seed production (Iinuma, 1967).
4. Japanese View of Composting: Tradition of the Supremacy of Mature Compost
Rice is the staple food of Japan. Rice is produced from rice, which is grown in paddy fields. Paddy fields have a wonderful mechanism whereby a significant portion of the nutrients absorbed by the rice can be recovered naturally after the rice is harvested. For example, the nutrients contained in irrigation water naturally replenish the rice. In addition, the watering of rice paddies changes phosphorus and iron into a water-soluble form, which is then absorbed by the rice plants. Thanks to a variety of other factors, the natural supply of nutrients in paddy fields is high. For this reason, the degree of nutrient wastage due to crop cultivation is smaller than in field soils. This can be understood from the fact that at the height of Norfolk agriculture in the 19th century, wheat seed yield was about 1.7 t/ha, while rice seed yield was already 1.8 t/ha at the end of the 16th century when the Taikoh land survey was conducted, almost equal to that of Norfolk agriculture (Takahashi et al. 1991).
In Japanese agriculture, livestock were mainly used as labor. Therefore, the number of livestock kept by farmers was small, and composts were generally produced by depositing ina straw or wheat straw and allowing them to ripen without including livestock manure. These composts, which do not contain livestock manure, are only fully ripened composts, and their effects as nutrients are difficult to be seen (the reasons for this will be explained later). The reason why the supremacy of fully ripe compost is emphasized in Japan is probably the result of the inheritance of such a tradition. We can sense the essential difference between paddy field agriculture in Japan and nutrient-recycling agriculture in Europe.
−第2章− 堆肥と化学肥料
第12回 堆肥の効果の現れ方と土の条件
−土の黒さが決め手−
令和4 (2022) 年 6月号 (第741号)
In the previous issue, I reviewed the history of how, in the days before the advent of chemical fertilizers, farmers overcame the difficult problem of supplying nutrients to farmland by devising a material called compost. I also mentioned the difference in the way of farming between the fields in Europe and the rice paddies in Japan, which led to a difference in the way of thinking about compost. Even after the advent of chemical fertilizers, compost is expected to have various effects. However, these various effects do not occur in all soil types without exception. Let us consider what are the conditions of soil that cause differences in the manifestation of these effects.
1. expected effects of compost
Compost is expected to have three major effects (Table 1). (1) as a nutrient, (2) as a stable organic matter that is relatively resistant to decomposition, and (3) as a source of living organisms. It is often said that composting will automatically produce all three effects at the same time. This is probably the reason why people think that compost improves the soil. However, these three effects are "expected effects" and do not always appear.
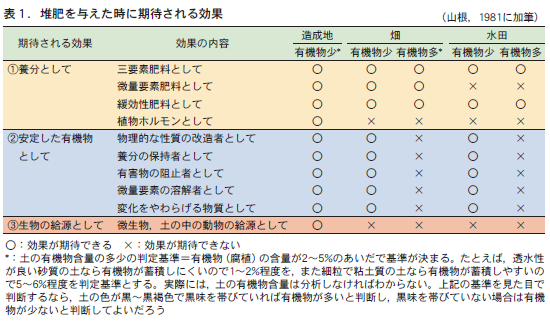
1)養分としての効果
まずは,養分としての効果である。堆肥を与えることで直接的に期待できる効果は,この効果である。具体的には,①多量要素,とりわけ三要素(窒素,リン,カリウム)の供給源,②微量要素の供給源,③ゆっくりと効果があらわれる肥料(緩効性肥料)としての効果,④植物ホルモンの供給源,などである。
これらの効果のうち,土の条件にかかわらず,堆肥を与えることで効果が確実に期待できるのは,①の三要素肥料としての効果である。通常の畑や水田の土で,窒素,リン,カリウムのいずれもが作物生産の制限因子とならないという土は考えにくいからである。また,③緩効性肥料としての働きも,土の条件にかかわらず期待できる。それは,堆肥が土に与えられた後,土の中の動物(トビムシ,ワラジムシ,ミミズなど)や微生物(細菌,放線菌,糸状菌など)などが協力して堆肥を分解し,その分解にともなって堆肥から養分が徐々に放出されるからである。堆肥を連用すれば,累積的で持続的な養分効果も期待できる。
However, the effect of (2) as a trace element fertilizer cannot be expected in paddy fields. This is because trace elements are dissolved in irrigation water in rice paddies, and the amount of trace elements supplied by irrigation water, which is taken up in large quantities during rice cultivation, is large. Even if the compost contains trace elements, the limited amount of trace elements supplied by the compost will not be equal to the natural supply from the irrigation water.
It is not yet well known how effective the plant hormones (4) can actually be in fields and rice paddies with a history of cultivation. However, it is likely to be effective when the surface soil containing organic matter is completely removed and the subsoil containing little organic matter is used as the cropping soil, as is the case in cultivated land.
(2) Effect as stable organic matter
The second expected effect is as stable organic matter. Stable organic matter is organic matter that remains in the soil after being decomposed to some extent by animals and microorganisms, and is relatively difficult to decompose. This becomes the substance known as soil organic matter (humus), which gives the soil its black color.
堆肥が土の中にすき込まれると,堆肥の中の分解されやすい有機物は,分解されることで養分的効果を現す。一方,比較的分解されにくいために土に残った有機物は,土の中にもともとあった有機物とともに安定した有機物としての効果を発現する。
その効果には,①土の物理的な性質,たとえば土のすき間の大きさやその割合(孔隙分布) ,排水のしやすさ(透水性) ,水持ちのよさ(保水性) ,空気の通りやすさ(通気性) ,耕しやすさ(易耕性)などの改良,②養分保持能の増加,③有害物の抑制,たとえば,有機物がアルミニウムと結合すると,アルミニウムの有害な働きを抑えるため(キレート作用という) ,アルミニウムがリンと結合しにくくなる。その結果,リンの養分効果が出やすくなるといった効果,④微量要素は水に溶けにくい形態であることが多い。しかし,有機物が分解されるにともない二酸化炭素(CO2)が放出され,これが水に溶けて炭酸水となって微量要素を溶けやすくする働き,さらに⑤有機物の持つ環境変化をやわらげる作用(緩衝力)などが考えられる。
However, these various expected effects of compost as stable organic matter appear only when the organic matter content of a given soil is less than a certain criterion (ranging from 2 to 5%, depending on the soil), and no effect can be expected if it is higher (Yamane, 1981). This is because in soils with high organic matter content, the physical properties of the soil are less likely to be a limiting factor in crop production, since the soil originally contains more stable organic matter (humus).
3)生物の供給源としての効果
三つ目の効果は生物の給源としての効果である。堆肥中には多くの生物(ミミズなどの小動物や微生物など)が生息している。堆肥を与えることは,土の中にこれらの生物を供給することになるので,その供給源としての効果が期待できる。
しかし,この効果も堆肥を与える土が通常の土であれば,その土に生息する生物数が,与えられた堆肥に含まれている生物数にくらべて圧倒的に多く,堆肥に土の生物の給源としての直接的な効果を期待しにくい。この効果も造成地のような極度に有機物の少ない土が作土となった場合に限定すべきである。
The effect of compost application on soil organisms is more likely to be cumulative than a one-year effect. However, even in this case, the direct effect of the diversification and increase in the number of organisms on crop growth may vary depending on other soil conditions.
2. the less organic matter in the soil, the more effective compost is.
When we give compost to the soil nowadays, are we just giving it to the soil without any particular reason, just because it is for "soil building"? We need to think carefully about why we are giving compost to the soil and what effect we expect it to have. Depending on soil conditions, compost may or may not have the desired effect. As shown in Table 1, the criterion is whether the soil has more or less organic matter.
The amount of organic matter in the soil can only be determined by strictly analyzing it. To determine the amount of organic matter without analyzing the soil, look at the color of the soil. If the color of the soil is black to dark brown with a black tint, you can judge that there is a lot of organic matter in the soil.
Soils with low organic matter (light black color) can expect diverse effects from compost feeding. For soils with high organic matter (dark black color), we should expect mainly nutrient effects as a slow-release three-element fertilizer.
−第2章− 堆肥と化学肥料
第13回 有機物資材の種類とその効果
−C/N比が要点−
令和4 (2022) 年 7月号 (第742号)
Compost was originally conceived as a material for transferring nutrients to farmland. In Japan, however, compost is now expected to be used as a stable organic matter and as a source of biological supply. Last month, we discussed the expected effects of such composts and how these expectations are reflected in crop production depending on the soil conditions.
This month, we will organize the expression of the effects of various organic materials, including compost, in terms of C/N ratio. Furthermore, in Japan, compost used is often limited to "fully matured compost. We will consider why this is so.
1. C/N ratio of organic materials and ease of decomposition
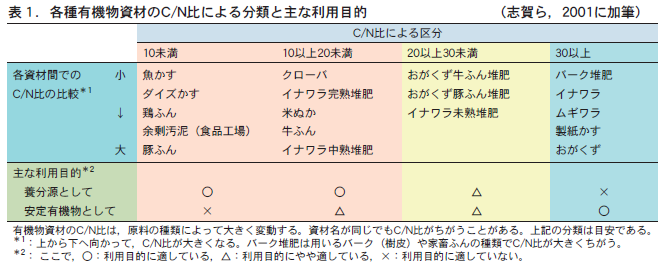
有機物資材が土に与えられると,土の微生物に分解されていく。この時,有機物資材の分解されやすさは炭素(C)と窒素(N)の比率(C/N比,炭素率ともいう)によって決まる。
炭素が少なく窒素の多いC/N比が20くらいより小さい有機物資材(表1参照,ダイズかす,鶏ふん,牛ふんなど)は,土に混和されると微生物に早く分解される。このため,養分的な効果が現れやすい。つまり,養分源としての利用に適した資材である。完熟堆肥といわれるのはこのようなC/N比を持っている。しかし,分解されやすいので土に有機物として残るのはわずかしかない。それゆえ,このタイプの有機物資材には土の中で安定した有機物としての効果は期待しにくい。このため,土の物理的な性質を改善するには適当な資材とはいえない。
Conversely, organic materials with a C/N ratio higher than about 30 (see Table 1), which are high in carbon and low in nitrogen (e.g., bark compost, inawara, wheat straw, etc.), decompose slowly in the soil. For this reason, they are not expected to be very effective as nutrients. However, organic materials accumulate in the soil as relatively stable organic matter. Therefore, these materials should be used when the objective is to improve the physical properties of the soil.
Specific examples of each type of organic material and their main uses are listed in Table 1.
2. use organic materials with high C/N ratio with caution
When organic materials with a C/N ratio of 30 or higher are used, even if the purpose of their use is to improve the physical properties of the soil, they may have a negative effect on crops. After a short time of feeding this type of organic material, the crop may show symptoms of nitrogen deficiency, such as yellowish-green leaf color and growth suppression. This phenomenon is called "nitrogen starvation. This is a strange phenomenon in which crops become nitrogen-deficient despite the application of fertilizer. The mechanism by which nitrogen starvation occurs is interesting.
C/N比の大きい有機物資材が土に与えられ,それが微生物に分解されていくとき,微生物にとってエネルギー源となる炭素(C)は有機物資材から十分に供給される。これに対し,微生物にとって重要なタンパク質の栄養源である窒素(N)の供給量は,炭素に比べ相対的に少ない。このため,この資材では微生物の窒素要求を満たすことができない。そこで,窒素を必要とする微生物は,土の中にもともとある無機態窒素(硝酸態窒素やアンモニア態窒素)や,肥料として与えられた無機態窒素を栄養源として体内に取り込み,自身のタンパク質などの有機態窒素に形態を変化させる(これを無機態窒素の有機化という) 。結果的に,土の中にあった無機態窒素が作物に利用しにくい形態に変化するので,作物に窒素欠乏症が現れる。
3. Why is the supremacy of mature compost in our country?
As mentioned in the 11th issue of this series (May issue), in Europe, compost is traditionally regarded as a nutrient transfer material. Keeping livestock is a prerequisite for compost production, and compost is regarded as a source of nutrients itself. Therefore, as in Japan, compost is not expected to have a variety of effects, including effects as a stable organic matter and as a source of biological resources, in addition to its effect as a source of nutrients.
On the other hand, before the advent of chemical fertilizers, compost had to be used as a source of nutrients in Japan. In Japanese agriculture, however, livestock were used mainly for labor, not for compost production. Farmers kept only a small number of livestock, and there was not much livestock manure available for compost production. Most of the raw materials for composting were ina straw, wheat straw, and fallen leaves and branches from nearby satoyama, and their C/N ratios were as high as over 60. If the C/N ratio remained high, the crops would be at risk of nitrogen starvation.
腐熟させる過程で,含まれていた炭素は,酸素が十分ある条件(好気的条件)で微生物によって分解され,最終的に二酸化炭素(CO2)に変化して大気中に放出される。これによって原料に含まれていた炭素量が少なくなる。一方の窒素は,その多くは腐熟化が進んでも原料に温存される。このため,腐熟化をすすめても窒素(N)量は大きく変化せず,炭素(C)量が減少するためC/N比が小さくなる。完熟堆肥とは,腐熟化でC/N比を小さくさせた堆肥のことである。
C/N比の小さい完熟堆肥が土に与えられると,微生物による分解が早く進む。分解とともに,この堆肥に含まれる窒素は作物が吸収しにくい有機態から吸収しやすい無機態のアンモニア態窒素に変化する。そして,これが養分として作物に吸収される。つまり,イナワラやムギワラといったC/N比が大きい有機物資材は,十分に腐熟させて完熟堆肥まで分解させないと,土に与えても養分としての効果が現れにくい。化学肥料のない時代,わが国でも堆肥は貴重な養分源だった。養分源として利用しようとするかぎり,C/N比を小さくしておくのが必須条件だった。わが国で「堆肥を使うなら,完熟堆肥でなければならない」という完熟堆肥至上主義が強調されるのは,上に述べたような伝統が現在まで受け継がれた結果だろう。
Current compost is treated as industrial waste
In Japan, the value of compost as a source of nutrients has declined since the introduction of chemical fertilizers as a nutrient transfer material. Labor-intensive compost production is no longer done, and compost is used less and less. Rice farmers, in particular, began to avoid applying compost to their rice paddies. This is because composted manure releases large amounts of nitrogen after the summer, resulting in high-protein rice and a significant decrease in eating quality.
Livestock farmers often run multi-cattle operations relying on imported concentrate feed for livestock feed. Livestock farmers often keep a large number of animals and depend on imported concentrated feed for livestock feed. The compost produced by livestock farms is treated as industrial waste and is not used effectively. In Japan, it is doubtful whether compost is being used properly, despite the importance of "compost for soil preparation.
−第2章− 堆肥と化学肥料
第14回 養分源が堆肥から化学肥料へ変化する時代
−その歴史的経緯−
令和4 (2022) 年 8/9月合併号 (第743号)
The significant effect of compost on crop production can be traced back as far as the time of Aristotle. It was not until the Norfolk farming in the 19th century that compost was actively used to transfer nutrients to farmland and to increase food production (see the May issue of this year's Newsletter). This month's story is about the shift from compost to chemical fertilizers as a source of nutrients. The trigger was the agricultural depression. What was the background?
1. what the Flemish saying teaches
ノーフォーク農法は,養分循環からたどりついた4年輪作(コムギ−飼料用カブ−オオムギ−アカクローバ)の農法であった(図1) 。家畜のエサとなる飼料作物(飼料用カブやアカクローバ)に土の中にある養分を吸収させ,そのエサを家畜に与えて,その家畜からふん尿という形態で養分を回収し,最終的にそれを堆肥にして人の食料生産の畑へ移転させるという循環型農業である。これは,イギリス・ノーフォークの対岸,ヨーロッパ本土のフランドル地方(現在のオランダ南部からベルギー西部,フランス北部地域)に古くから伝わる「飼料なければ家畜なし,家畜なければ肥料なし,肥料なければ収穫なし」との格言そのものだった。ヨーロッパの輪作で,作物生産を維持するための養分移転資材(肥料)として堆肥が大きな役割をはたしていることを,この格言は雄弁に語っている。
However, there is another point in this saying that should not be overlooked. The compost is a source of fertilizer, but it is also a source of fodder for livestock. As the Flemish proverb points out, compost cannot be produced as a source of nutrients without producing crops that feed livestock (forage crops) rather than food for people. That is why Norfolk Farming allocates half of the land area on the farm to fodder crop production and adds fodder turnips and red clover to the crop rotation. This has not only increased the number of livestock that can be raised, but also made it possible to keep the livestock in the barns through the winter. As a result, the recovery rate of livestock manure has increased, and the production of manure as a source of nutrients has increased. This has made it possible to increase the amount of manure applied to fields that produce food for people. This has allowed the yield levels of wheat and barley to nearly double at a stroke.

2. alternative sources of compost nutrients needed to escape agricultural depression
Until the use of chemical fertilizers became commonplace, this method of farming became widely popular because of its revolutionary high yields. In the 19th century, British agriculture entered a golden age at the height of Norfolk farming. However, this golden age did not last long. The Cereal Laws, which had previously restricted grain imports, were repealed, and inexpensive wheat was imported in large quantities from the United States and Canada. This caused a major blow to wheat cultivation in the U.K. and led to an agricultural depression. This depression began around 1875 and continued for about 60 years, although it was temporarily interrupted during World War I (McLean, 1991).
The agricultural depression was severe in the Norfolk region as well. In Norfolk farming, fodder turnips and red clover plots, which are grown to feed livestock, generate no direct income. They are grown for manure production as a source of nutrients. In order to get out of the recession, farmers have been tempted to stop the unprofitable forage production and grow cash crops to feed people. This would increase their profits. However, there was a problem. If they stopped producing fodder, they would no longer be able to feed their livestock, which would also mean that they would no longer be able to produce compost, and crop production itself would decline. Therefore, the important thing was to find a source of nutrients that could be substituted for compost. This is where chemical fertilizers, which were beginning to be sold and distributed at the time, came into the spotlight.
3. results of agricultural experiment stations established with farmers' investment
市販化学肥料として過リン酸石灰が世界で初めて登場したのは1843年7月1日。農業不況が始まる少し前のことだった。もちろん,化学肥料が一般の農家に広く使われるという状況ではない。化学肥料の普及にはまだまだ時間が必要だった。使用経験のない化学肥料が,本当に堆肥の代替養分源となるのかどうか,誰もが不安だった。それを解消するには科学的裏付けが欲しかった。そこで,ノーフォークの農家は自ら出資してノーフォーク農業試験場(後にモーレイ研究センターの時代を経て,現The Morley Agricultural Foundationに継承)を1908年に設立した(図2) 。

Results of a 12-year long-term crop rotation trial at the Norfolk Agricultural Experiment Station showed that when crop residues (e.g., wheat straw and sugar beet tops) are incorporated into the soil with chemical fertilizers, wheat and barley seedling yields can be maintained at the same level as in composted treatments, even without the application of compost ( Raynsand Culpin, 1948). Based on these results, it was recommended that sugar beets be grown instead of turnips for forage and that balayasho be grown instead of red clover (McLean, 1991), provided that chemical fertilizers were used in combination. Thus, concerns about chemical fertilizers were gradually resolved, and the source of nutrients for Norfolk farming gradually shifted from compost to chemical fertilizers, leading to the acceptance of chemical fertilizers in the world.
4. dilemma associated with compost production in recycling-oriented agriculture
The main reason for the introduction of chemical fertilizers into Norfolk farming was the need for land to produce fodder for livestock, which is the key to the nutrient cycle. The agricultural depression made it impossible to allocate that land for feed production. Moreover, as crop yields increase, the amount of nutrients taken out of the field also increases. This requires a large amount of compost as a source of nutrients to replenish the crop. Increased production of manure requires an increase in the number of livestock, as well as more farmland for the production of feed for the increased number of livestock. However, the profitability of that farmland is low. This is the dilemma that arises when trying to rely solely on compost as a source of nutrients in recycling-oriented agriculture.
More importantly, as Liebig, who with Sprengel argued that plant nutrients are inorganic, once criticized, there can be no perfect cycle in any cyclical agriculture. The produce is taken off the farm to be sold. The nutrients absorbed by the farmland are surely taken out of the circulation path. Unless the nutrients are brought in from outside the circulation path, the nutrients contained in the farmland in the circulation path are gradually depleted. Liebig called this plunder agriculture and recommended the use of chemical fertilizers to replenish nutrients.
5. another test to confirm fertilizer efficacy of chemical fertilizers
It was Rose of England who first introduced chemical fertilizers to the world. Rose was also concerned about whether chemical fertilizers were really as effective as compost. He therefore founded the Rothamsted Agricultural Experiment Station on his own to test the efficacy of fertilizers. This was 65 years before the Norfolk Agricultural Experiment Station, the oldest agricultural experiment station in the world. The testing began in 1843, the year chemical fertilizers were first sold. These tests have continued to the present day, some 180 years later. We will discuss the results in next month's issue.
−第2章− 堆肥と化学肥料
第15回 化学肥料だけしか使わない畑のコムギの生育
−堆肥だけの畑と比べる−
令和4 (2022) 年 10月号 (第744号)
化学肥料を世界で最初に市販したのは,イギリスのローズだった。1843年7月1日のことである。それまでの作物の養分源はもっぱら堆肥であった。ドイツのテーヤが指摘した植物の養分は土にある有機物(フムス=腐植)であるというのが通説の時代だったからである。
1. an era in which plant nutrients are changing from organic to inorganic
In 1828, Sprengel of Germany was the first to question this common theory and point out that plant nutrients are not organic but inorganic (minerals). It was Liebig, also of Germany, who further supported his point of view and popularized the theory in 1840. It was during this period that Rose marketed an inorganic chemical fertilizer (a patented fertilizer composed of lime superphosphate, ammonium phosphate, and potassium silicate) as a nutrient for crops.
Rose attempted several trials in his hometown of Rothamsted, Harpenden, to verify the efficacy of the chemical fertilizers he intended to sell. He conducted pot trials in 1837-39 and small field trials in 1840-41. From these trials, he recognized the importance of phosphorus as a nutrient for cabbage, since the highest yields were obtained when cabbage was treated with ammonium phosphate, which contained phosphorus as well as nitrogen.
2. overview of broadbore wheat field trials
Rose brought in Gilbert, who had studied chemistry at Liebig's, as a scientific collaborator and began comparing the nutrient effects of chemical fertilizers and compost. It was in the fall of 1843, the very same year that chemical fertilizers were introduced to the world, that he sowed wheat (fall-sown wheat) as a crop for testing. This is the Broadbark wheat test plot introduced this month. The year 1843 was the founding year of the Rothamsted Agricultural Experiment Station.
This field trial has continued uninterruptedly since then until now, 179 years later. In addition to the no-fertilizer treatment, the chemical fertilizer-treated area was given a certain amount of phosphorus, potassium, magnesium, and other nutrients in addition to nitrogen at four levels: 0, 48, 96, and 144 kg/ha of nitrogen. Currently, in addition to these four levels, trials are continuing at seven levels, including treatments of 192, 240, and 288 kg/ha. Of course, since the trials were started in the same year that chemical fertilizers were introduced to the market, there is no field on earth that has been grown with chemical fertilizers alone for a longer period of time than the Broadbork field.
1968年には,試験に用いるコムギの品種を高収量品種(稈長を短くして多肥条件でも倒伏しにくくし,葉を直立にして受光態勢を改良した品種)に変更している。同じ1968年からそれまでの連作処理の他に5年輪作の処理を加え,堆肥35t/haに窒素を96kg/ha(2005年からは144kg/haに増量)追加する処理もおこなうようになった。
3. results of broadbark wheat field trials
1)適量の化学肥料で堆肥区と同等の収量
Figure 1 shows the results of this trial. The yield of wheat seedlings in the chemical fertilizer (N144 kg/ha) area of the continuous wheat test was not much different from that in the compost area. It was confirmed that the production of wheat seedlings with chemical fertilizer alone was almost equal to that with 35 t/ha of compost, if the amount of fertilizer applied was appropriate.
Interestingly, after 60 years of continuous cropping, in 1902, both treatments showed continuous crop failure and yield decreased. When the fallow treatment was introduced (one-year fallow followed by four-year row cropping), yields recovered again. This indicates that the continuous cropping failure of wheat occurs not only in the chemical fertilizer area but also in the compost area, and that the fallow treatment is more effective for recovery than the nutrient treatments such as compost or chemical fertilizer.
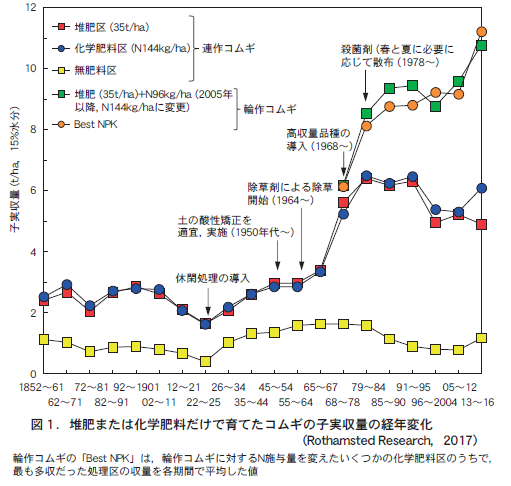
(2) Dramatic increase in yields with the introduction of high-yielding varieties
Since 1968, when high-yielding varieties were introduced, the yield of row crops of wheat has nearly doubled, despite no change in compost or chemical fertilizer application treatments. This confirms the high seed production capacity of high-yielding varieties.
Furthermore, in the five-year crop rotation established after 1968, when the nitrogen content of chemical fertilizers was added to the compost to increase the total nitrogen application, the yield was nearly 10 t/ha. This is not only about three times the yield of the composted area in the old variety era until 1967, but also about twice the average yield in Japan. The effect of the addition of chemical fertilizer nutrients to the compost is clear, and the high-yielding variety's response to fertilizer nutrients is understandable.
3) Soil organisms in fields where only chemical fertilizers are used.
化学肥料を使いつづけると土の中の生物が死に絶え,「土が死ぬ」と心配する人がいる。もし,化学肥料を使い続けることで土の生物が絶滅するのであれば,もちろん,この試験のコムギの収量や生育に影響するはずである。しかし,そのような現象は,世界で最も長く化学肥料だけを使い続けた,このブロードボーク・コムギ試験圃場でさえ,まったく認められていない(図1) 。化学肥料区と堆肥区の土の生物数を調査した結果によると,化学肥料だけを施与し続けたために,土の生物が生息しなくなったという事実はない(表1) 。
Russell, who was the head of the Rothamsted Farm Experiment Station for many years, clearly pointed out that "there is some concern that chemical fertilizers are harmful to earthworms and should not be used. However, Russell clearly pointed out that "there is no indication that this is the case in the Broadbark wheat test plots after more than 100 years of continuous application of chemical fertilizers in amounts greater than customary" (Russell, 1957).
These test results indicate that, as long as chemical fertilizers are used appropriately, there is no need to worry about their negative effects on crops.
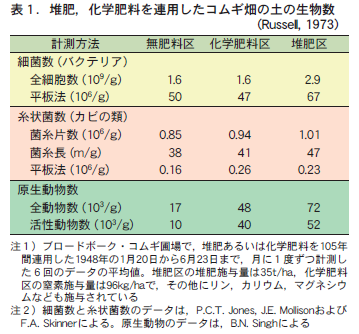
−第2章− 堆肥と化学肥料
第16回 堆肥と化学肥料,その効果を比べる
−共通点とちがいは何か−
令和4 (2022) 年 11月号 (第745号)
From the 11th (May) to the 13th (July) issue of this series, we have discussed compost, and in the 14th (August/September) and 15th (October) issues, we have discussed chemical fertilizers. We have described under what circumstances compost and chemical fertilizers were introduced to the world and what effects they have on crop production. In this month's issue, we will review the past, compare the effects of compost and chemical fertilizers, and summarize the similarities and differences between them. In the following, the term "compost" simply refers to organic materials, including both fully matured and immature compost.
1. effects of diverse composts
すでに第12回で詳しく述べたように,堆肥を農地に与えた場合,大きく分けて3つの効果がある。すなわち,①養分としての効果,②比較的分解されにくい安定した有機物としての効果,③生物の供給源としての効果である。ただし,これらの効果は,あくまでもこうした効果が期待されるということであって,堆肥を土に与えれば必ず自動的に発現するということではない。また,③生物の供給源としての効果は,新規に造成された農地のような栽培履歴がないところで発現する効果であり,栽培歴のある農地ではその効果に大きな期待はできない。
Furthermore, the effects of (1) and (2) may or may not be expected depending on soil conditions (organic matter content). Moreover, there are many kinds of composts. The effects that can be expected from various types of compost depend on the C/N ratio, which is the ratio of carbon (C) and nitrogen (N) contained in the compost.
Therefore, Figure 1 summarizes what kind of compost can be used on what kind of soil for farmland with a history of cultivation.
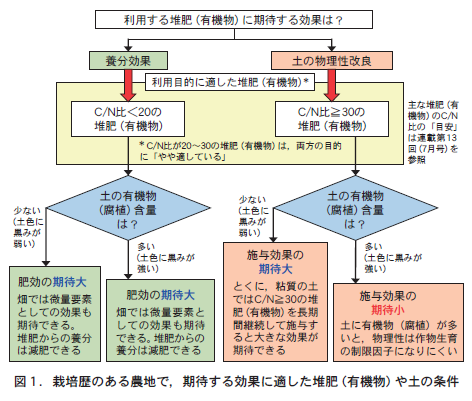
2. chemical fertilizers only have a nutrient effect
On the other hand, the effects of chemical fertilizers are not as diverse as those of compost. In other words, among the effects of compost, chemical fertilizers can only be expected to be effective as (1) nutrients. Only compost can be expected to improve the physical properties of the soil, such as the size and proportion of gaps in the soil (pore space distribution), ease of drainage (drainage property), water retention (water retention property), air permeability (aeration property), ease of cultivation (tillability), and various other effects as stable organic matter, as we pointed out in the 12th issue (June issue). The effects of compost alone can be expected only from compost.
3. compost and chemical fertilizers differ in the onset of nutrient effects.
The effects of compost and chemical fertilizers differ in their effects as nutrients. As a rule, the nutrients in chemical fertilizers are in an inorganic form that is easily absorbed by crops. Moreover, they are manufactured so that when they are applied to the soil, they dissolve in the soil moisture and are absorbed by the crop. Therefore, the nutrient effect of chemical fertilizers is characterized by rapid action. If a crop shows signs of nutrient deficiency during its growth, the delay in growth can be corrected with additional chemical fertilizers. This is possible because chemical fertilizers are fast-acting.
4. effect of stable organic matter on improving soil physical properties
On the other hand, the nutrients contained in compost are mostly in the organic form, with only a few in the inorganic form readily absorbed by crops. The organic nutrients are decomposed by soil microorganisms before they can be absorbed by the crop. Therefore, compost is a slow-acting nutrient that takes time to become effective. Therefore, it is not suitable for use as a fertilizer.
Of the nutrients contained in compost, the total amount of the fast-acting inorganic portion and the relatively easily degradable (readily degradable) organic portion can be expected to have a fertilizing effect on the soil in the same year they are applied to it. The rest of the organic portion, which takes more time to decompose (persistent), remains in the soil and is carried over to the next year and beyond as stable organic matter.
C/N比が20未満と小さい堆肥は,分解されやすい。そのため養分効果が強く表れる。逆にC/N比が30以上と大きな堆肥は,分解されにくく安定した有機物として土に残る。それが土の物理性改良の効果をもたらす。
5. chemical fertilizers have high nutrient content and are light in weight as materials
化学肥料と堆肥の養分について,もう一つ重要なちがいがある。それは一定の重さに対して含まれている養分量のちがいである。化学肥料は堆肥より養分含量が桁ちがいに多い。そのため,化学肥料のほうが労働時間当たりに与えられる養分量が多く,単位養分量当たりの運搬コストが安い。例えば,平均的な堆肥(牛ふん麦稈堆肥)とトウモロコシ用の化学肥料銘柄「S380」号を比較すると,それぞれ同じ1kgを土に与えたとしても,化学肥料は窒素(N)で堆肥の130倍,リンなら(P2O5として)60倍,カリウムでは(K2Oとして)25倍も多く与えられる(表1) 。
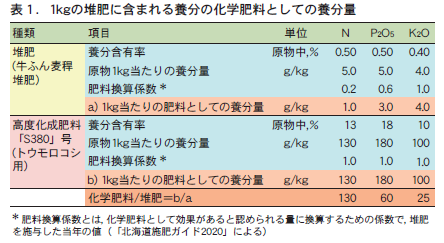
Because compost has a low nutrient content, the amount applied must be large in order to provide the large amount of nutrients needed to increase crop production. However, the amount is too large and labor-intensive. Chemical fertilizers, however, have made it possible to reduce this labor. This is because chemical fertilizers can provide large amounts of nutrients even in small amounts. Therefore, the use of chemical fertilizers has succeeded in greatly increasing the crop yield per area of land. Moreover, it also means an increase in production per labor hour. Thus, the production of food could be increased to support a large population.
The similarities and differences between compost and chemical fertilizers described above can be summarized in Table 2.
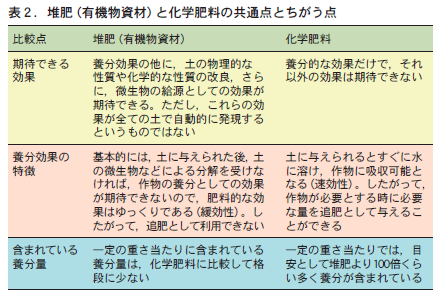
−第3章− 植物の養分吸収と吸収された養分の植物体内での働き
第17回 植物が水と養分を吸収するしくみ
-Absorb necessary substances and eliminate unnecessary ones.
令和4 (2022) 年 12月号 (第746号)
Plants absorb water and nutrients through their roots. However, the mechanism is not as simple as taking in substances dissolved in soil water (soil solution) into the roots at the same time as the solution.
1. plant cells surrounded by cell membrane and cell wall
Plant cells are surrounded by a plasma membrane, which in turn is surrounded by a cell wall (Figure 1).
細胞壁はザルのような網目構造で,水(ここでは,土壌溶液中の物質を溶かす溶媒としての純水H2Oのことを意味する。以下同様)はもちろん,土壌溶液に溶けている溶質としての養分イオンやその他の多くの物質も,自由に通過できる。細胞壁を通過した物質は,葉の蒸散作用によって作られる植物体内の水の流れに乗り,根の中心柱を取り囲む内皮(図1の④で,一つずつの細胞が輪をつくるようにつながっている組織)までたどり着く。これが図1の①の移動経路である。ここまではまだ細胞膜の外であり,水と養分イオンは細胞膜の内側に入っていない。
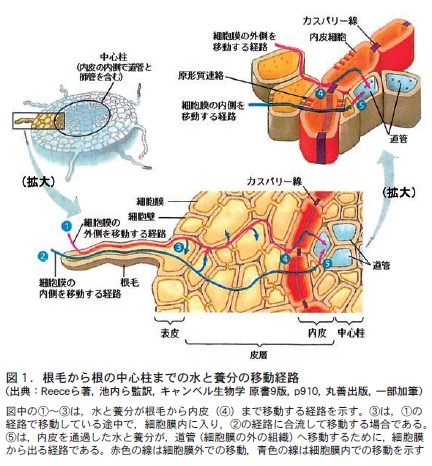
2.細胞膜内に入る最後の障壁
−カスパリー線
The cell wall of the endothelium is surrounded by a ribbon-like band of tissue called the cuspary line (Figure 1). Water and nutrient ions that have passed through the cell wall along the path shown in Fig. 1 (1) arrive at the cuspary line, where they are blocked and cannot move to the central column. This is because the cuspary line is made up of lignin, the main component of wood, and suberin, a type of lipid, and does not allow substances to pass through freely.
Apart from the pathway (1), there is another pathway where water and nutrient ions suddenly pass through the root hair cell membrane and move inside the cell (pathway (2) in Figure 1). In this case, the cell membrane that is first attempted to pass through determines whether it is allowed to enter the inside of the membrane. Only substances that are allowed to enter the membrane pass through the cell membrane. The next cell passes through the protoplasmic contact, which is a groove between cells. In this pathway, the endothelial cells can also pass through the endothelium and travel to the side of the central column ducts, since they have first entered the plasma membrane.
The problem is how it is allowed to enter the plasma membrane when it attempts to pass through the endothelial cell caspary line in pathway (1) and when it first passes through the plasma membrane in pathway (2), respectively.
3. cell membrane function and water absorption
-Osmotic pressure and transport proteins (aquaporins)
The cell membrane is composed of a bilayer of phospholipids consisting of hydrophilic and hydrophobic groups with hydrophobic groups next to each other (Figure 2). As shown in the figure, there are membrane-spanning proteins, which together constitute the cell membrane. These membrane-spanning proteins are called transport proteins, and play a major role in the transport of substances across the plasma membrane.
この細胞膜は細胞内の様々な物質を抱えているので,一般に,物質濃度は細胞膜内で高く,膜外は低い。細胞膜が細胞壁のように,すべての物質の通過を許す全透膜であれば,通常は濃度勾配による拡散によって,濃度の高い細胞内から濃度の低い膜外に養分イオンが出ていく。これでは,養分イオンが養分として利用できなくなる。
このため,細胞膜は溶媒の水を通過させるが,溶質の養分イオンなどの物質を通過させない半透膜になっている。この時水は,細胞膜の内外の濃度差を消すように,高濃度の細胞膜内側に入り込んで吸収される。この半透膜の内外の濃度差で発生する圧力が浸透圧である。ところが,浸透圧だけの水の移動速度は遅く,植物の水要求を十分に満たせない。それを補う水の輸送タンパク質がアクアポリン(水チャネルともいう)で,水の輸送速度は非常に速い。多くの植物細胞では,アクアポリンによって水の輸送速度が10倍以上高まる(平沢,2016) 。植物が土壌溶液から水を吸収するのは,主にアクアポリンの働きである。
4.養分が細胞膜を通過するしくみ
−輸送タンパク質による能動輸送
Transport proteins are also responsible for the entry of nutrient ions dissolved in the soil solution into the plasma membrane. Nutrient ions, which are solutes in the soil solution, cannot pass through the cell membrane, which is a semipermeable membrane. Moreover, since there is a concentration gradient between the inside and outside of the plasma membrane, nutrient ions must enter the plasma membrane against the concentration gradient in order to be absorbed. This is made possible by the function of transport proteins that penetrate the plasma membrane.
These transport proteins do not freely transport any substance, but have their own specific transport partners. As already mentioned, water is transported by aquaporin, a water-specific transport protein. Ammonium ions, the nutrient ions of nitrogen, have a transport protein (ammonium ion transporter) that is responsible for their passage through the membrane. Plants are able to selectively take up only the nutrient ions they need from a soil solution containing various substances (selective absorption) because each transport protein has the property of transporting its own counterpart. At this time, there are also transport proteins that generate energy to transport nutrient ions from the outside to the inside of the membrane against the concentration gradient (active transport).
Thus, plants skillfully selectively absorb only water and nutrient ions from substances dissolved in the soil solution around their roots, eliminating unwanted substances.
5. the final step in nutrient absorption is transfer to the ducts
Absorbed water and nutrient ions do not remain in the root cells. The absorbed water and nutrient ions are transferred to the various parts of the plant body, such as the stem and leaves. This is done through ducts. Since ducts are pipes for transport, they are the outer tissues of the plasma membrane and consist mainly of cell walls.
Therefore, water and nutrient ions that enter the endothelial cell must again move out of the plasma membrane and into the ducts (Fig. 1, movement ⑤). The transport protein that performs this role is often not the same transport protein that was used to enter the plasma membrane, but another transport protein.
Thus, water and nutrient ions that reach the ducts move to the plant organs where they are needed, where they are used as nutrients and converted into substances that nourish the plant.
−第3章− 植物の養分吸収と吸収された養分の植物体内での働き
第18回 植物が難溶性物質を吸収するしくみ
−根から溶解を助ける物質を分泌する−
令和5 (2023) 年 1月号 (第747号)
Plants select and absorb the nutrients they need from the water (soil solution) in the soil. In the last issue, I explained how this works. If nutrients are easily soluble in soil solution, it was explained. But what do plants do when nutrients exist in the soil as insoluble substances that are difficult to dissolve in water?
In this article, I would like to look at the amazing mechanism by which plants absorb nutrients from substances that are difficult to dissolve in soil solution.
1. phosphorus and iron are insoluble substances in the field
Among plant nutrients, phosphorus and iron exist in the soil in the form of substances that are insoluble in water (insoluble substances, such as aluminum phosphate, iron phosphate, and iron hydroxide). However, when the soil surface is covered with water and the soil is in a reduced state due to lack of oxygen, as in paddy fields, the insoluble iron phosphate and iron hydroxide are transformed into water-soluble substances. Therefore, rice plants are seldom significantly deficient in these nutrients.
On the other hand, in field soil, oxygen is connected to the atmosphere and remains in an oxidized state. For this reason, phosphorus and iron exist as insoluble substances, which are difficult to dissolve in soil solution, making them difficult for plants to absorb. However, plants absorb such nutrients by taking measures as described below.
2. mechanism of phosphorus absorption from insoluble phosphorus
In field conditions, phosphorus is often present as the insoluble substances aluminum phosphate and iron phosphate. In addition, phosphorus may also be present in the form of organic phosphorus, which cannot be absorbed without modification. To facilitate the absorption of such water-insoluble phosphorus, plants produce organic acids (citric acid, oxalic acid, piscic acid, etc.) and an enzyme called acid phosphatase in the root cells and discharge them into the soil around the roots (this phenomenon is called secretion) (Figure 1). Of course, these secreted substances must also pass through the cell membrane to exit the root. The transport of these substances is handled by the respective transport proteins (transporters).
Organic acids secreted by the roots have the ability to dissolve iron phosphate and aluminum phosphate in the soil by stripping their bonds. The dissolved phosphorus in ionic form is transported into the cell membrane through transport proteins and absorbed by the plant. The iron and aluminum that are stripped from their bonds do not remain in the soil solution as ions. They are transformed by organic acids into an encapsulated form (this kind of reaction is called chelation, and the resulting substance is called a chelating substance), which prevents them from rejoining phosphorus.
Acid phosphatases act on the organic phosphorus present around the roots and deliver water-soluble phosphate ions to the soil solution by enzymatic degradation (Figure 1). The delivered phosphate ions are absorbed into the cell membrane through transport proteins as plant nutrients.
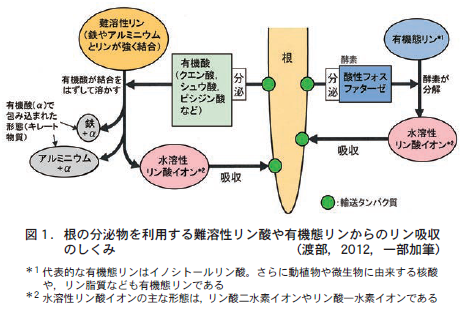
3. expand rooting to absorb phosphorus
Since phosphorus exists as an insoluble substance, the concentration of phosphorus in the soil solution is low. Therefore, plants sometimes try to absorb phosphorus in low concentrations by increasing root growth and expanding the root surface area. A similar mechanism is used by filamentous fungi (a member of the fungus family, mycorrhizal fungi) that live symbiotically in the roots. Mycorrhizal fungi spread their mycelium widely in the soil, take up phosphorus from the soil solution, and provide it to the host plant, which in turn supplies phosphorus to the plant.
4. two mechanisms of iron absorption from insoluble iron
畑状態の鉄は難溶性の酸化鉄(これは,鉄のサビと同じ物質。この鉄の形態は,三価鉄・Fe(Ⅲ)・Fe3⁺)で土の中に存在し,土壌溶液には溶けにくい。このままであれば,植物は鉄を吸収しにくく欠乏してしまう。しかし,畑状態で生育する植物は,リンの場合と同じように根から難溶性の鉄(三価鉄)を溶かす物質を分泌することで鉄を吸収している。ただし,イネ科以外の植物とイネ科植物とでは,鉄吸収のしくみが大きくちがう(図2) 。
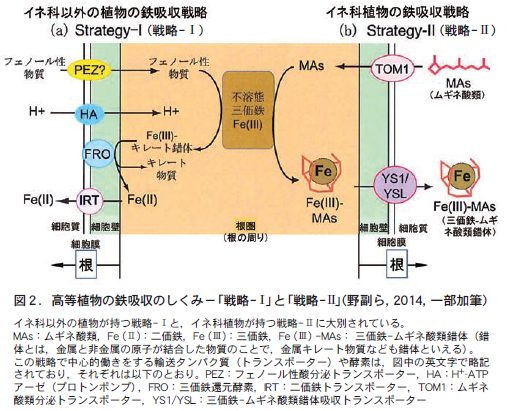
(1) Mechanism of iron absorption in plants other than grasses
これはStrategy−Ⅰ(戦略−Ⅰ)といわれる(図2の左側) 。まず,難溶性鉄(三価鉄)を溶かすゆるいキレート物質(フェノール性酸)を根から分泌する。この物質で三価鉄を包み込んで(キレート化して)細胞壁に持ち込む。すると,細胞膜表面に存在している酵素(三価鉄還元酵素,FRO)が働き,三価鉄を二価鉄(Fe(Ⅱ)・Fe2⁺)に変える。そしてこの二価鉄の輸送タンパク質(IRT)によって細胞膜内に吸収される。
Other mechanisms utilize trivalent iron, which is more soluble in acidic conditions. There is a transport protein (proton pump, HA) that releases hydrogen ions from root cells to the outside of the root. This proton pump lowers the pH around the roots, making it easier for trivalent iron to dissolve in water and be absorbed.
(2) Mechanism of iron absorption by rice plants
This is known as Strategy-II (right side of Figure 2). Organic acids such as mugineic acids and their analogues (MAs in the figure, see note in Figure 2 for explanation of the English letters), which are produced by the plant inside the root cells, are secreted outside the cell wall around the root through a transport protein (TOM1). This acts on trivalent iron, which is transformed into a substance (chelate called Fe(III)-MAs) encapsulated in mugineic acids. The chelates made from the iron and mugineic acids are absorbed into the plasma membrane by transport proteins (YS1 and YSL) responsible for their transport (Nozoe et al., 2014).
This mugineic acid was discovered by Professor Seiichi Takagi of Iwate University in Japan in 1976. Until then, the mechanism of iron absorption by plants other than grasses could not fully explain the iron absorption of grasses. However, this discovery clarified the mechanism. This was truly a major historical discovery.
−第3章− 植物の養分吸収と吸収された養分の植物体内での働き
第19回 吸収された窒素がタンパク質になるまで
−植物は必要なアミノ酸をすべて自給する−
令和5 (2023) 年 2/3月合併号 (第748号)
Plants select and absorb only the nutrients they need from the water in the soil (soil solution) by a well-developed mechanism. The absorbed nutrient ions support the nutrition of the plant by either becoming components of substances that help to nourish the plant or by participating in various reactions in the plant body.
This month, as an example, we will look at how plants use nitrogenous nutrient ions such as ammonium and nitrate ions and carbohydrates produced by photosynthesis in leaves to make proteins.
1. how amino acids are synthesized from ammonium ions
アンモニウムイオン(NH4⁺)の窒素(N)や水素(H)は,タンパク質の構成成分で植物の重要な養分である。しかし,アンモニウムイオンが多量に植物の地上部に送り込まれると光合成を妨害するなど,植物に悪影響を与える。このため,多くのアンモニウムイオンは根の細胞内にとりこまれると,すぐにアミノ酸に合成されて無毒化される。その経路を示したのが図1である。
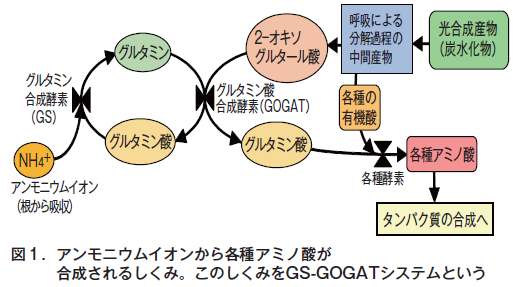
植物の根の細胞にとりこまれたアンモニウムイオンは,まずグルタミン酸というアミノ酸と結合してグルタミンというアミノ酸になる。この反応はグルタミン合成酵素の働きである。このグルタミンは,光合成でつくられた炭水化物が,植物の呼吸によって分解される過程の中間産物である2−オキソグルタール酸という有機酸と反応して,二つのグルタミン酸に変わる。この反応は,グルタミン酸合成酵素の働きである。
Of the two glutamic acids, one is again combined with ammonium ions to make glutamine. The other reacts with various organic acids, which are intermediate products of the breakdown of photosynthetic products by plant respiration, to produce the necessary amino acids. In this case, the reactions are also carried out by enzymes that assist in each reaction. In this way, all necessary amino acids are self-sufficient, and proteins are synthesized from them.
2. how amino acids are synthesized from nitrate ions
アンモニウムイオンは図1の経路でアミノ酸合成がおこなわれる。ところが,アンモニウムイオンは,畑のように空気中の酸素に触れやすい条件(酸化条件)では,土の微生物の働きで硝酸イオンに変化する。これが硝酸化成作用である。したがって畑作物などが吸収する養分としての窒素の形態は硝酸イオンが主体である。この場合,どのようにしてアミノ酸が合成され,タンパク質の材料となるのだろうか。ここでも植物はたくみなしくみを用意している。
植物に吸収された硝酸イオンがアミノ酸の原料となるには,硝酸イオンがアンモニウムイオンに変化し,図1のアンモニウムイオンからアミノ酸合成されるしくみ(GS-GOGATシステムという)に組み込
まれていく必要がある。その働きをおこなうのが硝酸還元酵素と亜硝酸還元酵素である(図2) 。いずれも酵素反応で,協同して硝酸イオンをアンモニウムイオンへ変化させる。
多くの硝酸イオンは根で吸収されると,そのままの形態で道管を通って葉へ移動する。葉は日光によく照らされ,この酵素反応に必要な光エネルギーを獲得しやすいからである。葉へ移動した硝酸イオンは,硝酸還元酵素の働きで亜硝酸イオンに変化する。さらに亜硝酸イオンは,亜硝酸還元酵素でアンモニウムイオンに変化する。そして,アミノ酸合成経路のGS-GOGATシステムに入り(図2) ,必要なアミノ酸合成がおこなわれる。
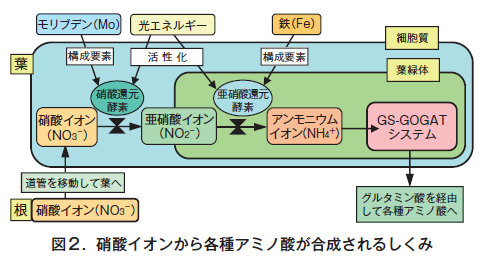
3. mechanism to prevent excessive accumulation of ammonium ions
The issue here is the relationship between the rate of the enzymatic reaction that converts nitrate to ammonium ions and the rate at which the resulting ammonium ions are incorporated into the GSGOGAT system for amino acid synthesis. If the rate of the former exceeds that of the latter, the ammonium ions produced by this enzymatic reaction will accumulate in the leaves. However, this must be avoided. The accumulation of ammonium ions is detrimental to the plant. To avoid this, nitrate reductase has a function that prevents the accumulation of ammonium ions.
硝酸還元酵素は硝酸イオンが吸収されることで酵素反応を活性化させ,亜硝酸イオンをつくる。しかし,この亜硝酸イオンが亜硝酸還元酵素の働きでアンモニウムイオンになると,それがアミノ酸合成に組み入れられて細胞内で無毒化されるまで,硝酸還元酵素は自身の酵素活性を抑制する。つまり,硝酸還元酵素はむやみにアンモニウムイオンをつくらないように酵素活性を自己規制している。これによって,硝酸イオンがアンモニウムイオンに変換される速度と,酵素反応でできたアンモニウムイオンがアミノ酸へ変換される速度との間でバランスが維持されている。この硝酸還元酵素のように,状況に応じて目的にかなうように反応活性の調節機能を持つ酵素を適応酵素あるいは誘導酵素という。
The fact that nitrate reductase is an adaptive enzyme is especially important for many plants that mainly absorb nitrate as a nitrogen nutrient source, in order to avoid the danger of excessive accumulation of ammonium ions.
4. the only external raw material required for amino acid synthesis is ammonium ions
From what we have seen so far about the mechanism of amino acid synthesis, we can notice something. The only raw material that plants need to acquire from outside in order to synthesize amino acids is ammonium ions. The other raw material, photosynthetic products (carbohydrates), is synthesized by the plant itself. This is made possible by two enzymes that synthesize glutamine and glutamic acid. It is thanks to the action of these two enzymes that plants are able to synthesize complex amino acids from simple substances such as ammonium ions.
5. plants do not contain essential amino acids
私達は,タンパク質の合成に必要なアミノ酸のすべてを自給できない。そのため,食べものから必要なアミノ酸を獲得する必要がある。これが必須アミノ酸である。植物はタンパク質の合成に必要なすべてのアミノ酸を自給するので,植物に必須アミノ酸はない。動物の必須アミノ酸のような物質が植物にもあるというような誤解は避けるべきである。
−第3章− 植物の養分吸収と吸収された養分の植物体内での働き
第20回 農産物のおいしさに影響する
タンパク質と炭水化物はトレードオフの関係
令和5 (2023) 年 4月号 (第749号)
In the previous issue, we introduced the GS-GOGAT system, in which plants absorb ammonium and nitrate ions, which are nitrogenous nutrient ions, and utilize carbohydrates produced by photosynthesis in leaves to produce amino acids, which are the raw materials for proteins. Using this system, plants supply themselves with all the amino acids necessary for protein synthesis. Proteins, along with carbohydrates, greatly affect the taste of agricultural products.
In this article, we will consider the curious trade-off between protein and carbohydrate content in crops, which has a significant impact on the taste of agricultural products, from the perspective of the GS-GOGAT system.
1. what is a trade-off relationship?
A trade-off is a relationship in which one factor increases while the other decreases, like a seesaw on a playground, and the two factors cannot both increase and be compatible. For example, as shown in Figure 1, when the nonstructural carbohydrate (NSC) content per panicle of rice is low, the protein content in brown rice is high, and when the NSC content is high, the protein content in brown rice is low, indicating that a trade-off relationship exists between the two.
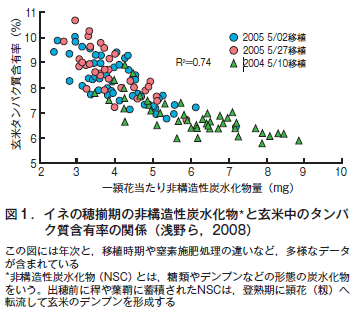
2. why the trade-offs?
植物体内で,タンパク質と炭水化物の含量がトレードオフの関係になるのは,植物体内の炭水化物含量 (A) が,光合成による炭水化物生産量 (B)と吸収された窒素が体内でタンパク質に変換されるときに消費される炭水化物量 (C) との差で決定されるから(A = B−C)である。
This can be well understood from the perspective of the GS-GOGAT system introduced in the previous issue. That is, plants that are given a lot of nitrogen from compost or chemical fertilizers absorb a lot of nitrogen, of course. When more nitrogen is absorbed, it is incorporated into the GS-GOGAT system and amino acid synthesis is activated, resulting in an increase in protein content.
この時,アミノ酸合成のための原料は,根から吸収するアンモニウムイオンや硝酸イオンの他に,もう一つの原料が必要だったことを思い出してほしい。それが2−オキソグルタール酸である。アミノ酸合成を活発化させるには,この2−オキソグルタール酸を多量に供給する必要がある。2−オキソグルタール酸は,葉でつくられた光合成産物の炭水化物を,植物の呼吸作用で分解する過程でできる中間産物の有機酸である。それゆえ,この有機酸を多量に供給するには,呼吸作用を活発にして炭水化物の分解を多くしなければならない。その結果,植物体内に残る炭水化物量は必然的に
少なくなる。
Conversely, if only a small amount of nitrogen is provided, plants synthesize only a few amino acids and have low protein content. Since not much is used for amino acid synthesis, not much is needed for the intermediate products of carbohydrate breakdown by respiration. As a result, more carbohydrates are left over, resulting in a relatively high carbohydrate content. This is the main mechanism that causes the trade-off relationship between protein and carbohydrates in plants.
Nitrogen fertilization and crop quality
The trade-off between protein and carbohydrate content in crops means that nitrogen fertilization has a significant effect on the carbohydrate content of crops. We would like to consider this relationship in terms of the eating quality of rice and the relationship between different cultivation methods and the taste of the crop.
1) Protein content and eating quality of rice
コメ(精米)の食味総合評価は,明らかにタンパク質含量が低いほど高い評価を受ける(図2)。これは,低タンパク質含量であるほど炭水化物(デンプン)含量が高まり,日本人好みの食味になるからである。したがって,コメの食味を良くするために,イネのタンパク質含量を必要以上に高めない窒素の肥培管理が求められている。しかし,低タンパク質含量をねらうあまり,窒素施与量を少なくしすぎると玄米収量が低下する。つまり,高デンプン質の良食味米を生産するのは,食味と収量のギリギリのバランスで,窒素の肥培管理をおこなう高い技術を必要としている。
However, the eating quality of rice (milled rice) is not only affected by protein content, but also by low amylose content, which constitutes the starch of rice. The amylose content of rice is more influenced by variety characteristics than by nitrogen fertilization. Therefore, good-tasting rice varieties are selected from breeding material that has characteristics that result in low amylose content in the rice.
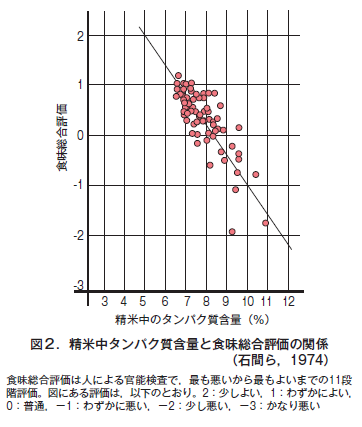
(2) Differences in cultivation methods and the taste of agricultural products
It is often pointed out that organically grown produce tastes better than conventionally grown produce (conventionally grown using chemical fertilizers and pesticides). Is this a general fact? This can also be explained in terms of the relationship with the amount of nitrogen applied.
Compost is used as the nutrient source for the crop in organic farming, while chemical fertilizer is used in conventional farming. Let us now consider a case in which crops are grown using these two sources of nutrients with the same amount of total nitrogen applied. Even if the amount of total nitrogen applied is the same for both sources, the amount of nitrogen in a form that is easily absorbed and utilized by the crop (inorganic nitrogen) is usually much higher with chemical fertilizer than with compost. This is because the nitrogen in compost contains organic nitrogen that cannot be immediately absorbed by the crop.
いいかえると作物からみれば,与えられた全窒素量が同じでも,すぐに吸収できる無機態窒素量は慣行栽培の化学肥料で与えられるほうが,有機栽培の堆肥で与えられた場合より多い。そうすると,慣行栽培のほうが有機栽培よりも作物の窒素吸収量が増えて,タンパク質含量が高まり,その結果,炭水化物含量が低くなる。農産物のおいしさは,糖類やデンプンなど炭水化物含量が多いほど高まると考えられるため,結果的に与えた全窒素量が同じなのに有機農産物のほうがおいしいという評価になる。しかし,それは栽培法のちがいに由来することではなく,用いた養分源に含まれる無機態窒素量のちがいが主な原因である。化学肥料からの窒素施与量を,堆肥に含まれている無機態窒素量と等しくすれば,作物の糖類などの炭水化物含量に,両者間で大差なくなる。
In other words, the fact that organic produce generally has lower protein content and higher carbohydrate content than conventional produce is not due to differences in cultivation methods, but rather to differences in the amount of inorganic nitrogen applied, even when the total nitrogen content fed is the same.
−第4章− 土のでき方と地球上での役割
第21回 「土は生きている」といわれるのはなぜ?
−土は生き物なのか
令和5 (2023) 年 5月号 (第750号)
In this issue, we turn our attention to the soil itself. It is the soil that stably supports agriculture. The soil is the place where food that protects our lives is produced, and at the same time, it nurtures the lives of various living creatures. For this reason, it is often said that "the soil is alive" in awe of it. Why is this so?
1. Is soil a living thing?
土が本当に「生きている」 ,すなわち生き物なのかと改めて問いただされると,土が生き物であるという人は多くはないだろう。
一般的な概念で生き物というのは,多細胞生物である。その多細胞生物であるためには,①分化と生長,②繁殖と遺伝,③環境変化への自律性を満たす必要がある(岡島,1989) 。しかし,土に両親がいて,その遺伝的要素を引き継いで大きくなり,子供を育て,死んでいくとはだれも考えないだろう。「土は生きている」というのは,あくまでも,土を譬喩(ひゆ)的に表現しているにすぎない。土を敬愛するあまりに,土が生き物であると混同するのはつつしむべきだろう。
But even if it is a parable, the expression "the soil is alive" does not fail to capture our hearts. Why is that?
2. soil has properties similar to the autonomy of living things
Soil has a property that gives a sense of the autonomy that living things have over their environment, i.e., the ability to maintain their own state of health in the face of external stimuli. This is the buffering power of soil.
具体的に土の緩衝力を見てみよう。土に酸性物質として塩酸,アルカリ性物質として水酸化ナトリウムをそれぞれ滴下する。これによって,土は外部から刺激が加えられたことになる。この時,図1に示したように,純水(H2O)の場合,ほんのわずかな滴下量で,pHが酸性側にもアルカリ性側にも大きく変化する。一方,土Aと土Bのどちらも,純水より変化の幅が小さい。つまり,土は純水より緩衝力が大きいのがわかる。
ただし,有機物の少ない土Aは,有機物の多い土Bよりも変化の幅が大きく,したがって緩衝力は小さい。土の有機物は,酸性の原因である水素イオン(H+)やアルカリ性の原因である水酸イオン(OH−)を静電気的に保持する能力を持つ(土の静電気的なイオン保持能については,昨年の2月号の第9回を参照)。このため,有機物がそれらのイオンを保持して動きを抑え,外界からの刺激をやわらげることができる。土Bのほうが土Aよりも緩衝力が大きいのは,土の有機物含量の違いに基づく。このような土が持つ緩衝力は,生き物の自律性とよく似ている。
However, the buffering capacity of the soil is not the only reason why the soil is said to be "alive. The activities of living creatures living in the soil are often invisible to our eyes, and appear to us as if they were performed by the soil itself. This is another major factor that makes us feel that the soil is alive.
3.生き物たちの生活の証し−土壌呼吸
The fact that compost fed to the soil becomes nutrients for crops, and that leaves that fall on the soil in the fall and kitchen scraps disappear eventually if they are buried in the soil, are all the result of decomposition of organic matter by soil organisms.
土の生き物たちが,堆肥や落ち葉,生ゴミなどの有機物を分解すると,分解産物の一つとして二酸化炭素(CO2)が排出される。この様子は動物の呼吸に似ていることから,「土壌呼吸」といわれる。あたかも土が呼吸しているかのような感覚である。しかしこれは,あくまでも土の中で生活している生き物たちの活動の証しであって,土自身が呼吸しているわけではない。
4. organic matter decomposition is a cooperative play among soil organisms
上で述べた落ち葉を例に,土の生き物たちが有機物を分解する時の,みごとな連係プレーを見てみよう(図2) 。
土の表面にある落ち葉(植物遺体)は,そのまま乾いた状態では大きく変化しない。しかし,そ
れがひとたび雨に打たれて濡れると,そこにまずバクテリア(細菌)やカビ(糸状菌)がとりつき,
この植物遺体をある程度軟らかくする。
Then, large soil animals such as earthworms, borers, and sowbugs appear and feed on the plant remains, pulverizing them and dragging them and their remains into the soil. Medium-sized soil animals, such as mites and stoneflies, then take charge, feeding on the dragged organic matter and taking nutrients into their bodies, and excreting the unwanted material as feces.
Bacteria and fungi feed on the excreted feces and the remains of plants that have been dragged into the soil, eventually transforming them into carbon dioxide, water, and inorganic substances. The inorganic matter produced by this process is absorbed by the roots as nutrients for the plants growing there. Thus, a cycle of nutrients is established. Of course, the same mechanism applies to animal remains as it does to plants.
The various material changes that occur in the soil include changes involving soil organisms. Therefore, when we talk about material changes in the soil, if we blur the distinction between the function of the soil and the function of living creatures in the soil, we confuse the two.
5. distinguish between the workings of the soil and the workings of living things.
土の働きと表現しても,それは土に生き物のような「意志=合目的性」があって,土自身の意志が能動的に働くことを意味していない。「土が作物に養分を供給する」という表現も実は不適切で,土自体が作物へ意図的に養分を供給することはない。土の中の水(土壌溶液)に溶けているさまざまな養分イオンの中から,作物が必要とする養分,例えば陽イオンの養分を選択して吸収すると,それまでの電気的中性原理(陽イオンと陰イオンの荷電量が等しい状態)のバランスがくずれる。くずれたバランスをもとの状態に戻すように,土の中で多様な反作用がくり広げられ,電気的中性原理を維持するために陽イオンが土壌溶液に放出される。これが「土が作物に養分を供給する」と表現される現象である。この反作用も一定の原理(この例では溶液での電気的中性原理)によって律せられており,生き物がもつ自由な意志がはいり込む余地はない。
I would like to understand the true meaning of the parable "the soil is alive" after distinguishing the function of the soil and the creatures in the soil.
−第4章− 土のでき方と地球上での役割
第22回 地球上の生命を育み,
地球環境を保全する土の役割
令和5 (2023) 年 6月号 (第751号)
前回は土の中の生き物についてお話しした。しかし,土は土の生き物だけに生活の場を提供しているのではない。地球の陸地に存在する生命のすべては,直接あるいは間接的に土の恩恵を受けている。それは,土で生育する植物を起点に,地球上での「捕食(食べる) −被食(食べられる)」という食物連鎖によって,多くの生命が養われていることから理解できる。それだけでなく,陸上生物の生活環境となる地球環境の保全にも大きな役割を果たしている。
In this article, I would like to consider these important functions of soil.
1. three major functions of soil
土の機能を概観すれば,道路や鉄道,建物などの基盤,各種の建築資材や窯業の原料,景観の構成要因といったことが思い浮かぶ。しかし,それ以上に,①陸上の植物を育てる機能(生産機能) ,②水を保持する機能(保水機能) ,③有機物や化学物質を分解し浄化する機能(分解浄化機能)で,地上の生命を支えるという重要な機能がある。
(1) Function of growing plants on land (production function)
Plants receive heat and light from the sun, carbon dioxide from the atmosphere, and water from the soil for photosynthesis. This plant is the starting point of food for at least all terrestrial organisms. The food of the creatures in the soil is also originally organic matter produced by plants. Carnivores are also able to eat meat because herbivores live on plants. Since the soil supports the growth of plants, the productive function of the soil is extremely important for terrestrial organisms.
土を利用せず,光,日長,温度などの生育環境を人工的に管理して作物を水耕などで栽培する場合がある。このような施設を植物工場ということがある(図1) 。
These cultivation methods require large energy costs for facilities and machinery for nutrient management, as well as for heating, cooling, and lighting, and high maintenance and management costs, including labor. In addition, the spread of external pathogens and diseases during cultivation is much faster than with soil cultivation. Furthermore, it is unrealistic to leave food production to hydroponics, considering concerns about the sustainability of fossil energy. The dependence of terrestrial organisms on soil for food production is unlikely to change in the future.

(2) Function to retain water (water retention function)
When the soil is covered with asphalt-paved urban streets and concrete buildings, much of the rainwater does not percolate into the soil, but instead flows directly into rivers through the sewage system. Under these conditions, when temporary heavy rains occur, small urban rivers overflow and cause damage. This is because the soil has lost its function of holding water.
土の保水能力の低下に起因する現象は,たとえば,森林を伐採して切り開いた丘陵地の宅地造成地でも認められる。そのような現場では,雨水が土の表面をかけ抜け,表土を削りながら濁流になって河川に流れ込む。土砂崩れも起きやすい。
21世紀は水問題の世紀といわれる(ポステル,2000) 。農地の食料生産でも,淡水資源が制限因子になって増産の期待がもてない。土の保水能力の低下だけでなく,地下に貯留されていた水(地下帯水層)の過剰利用で,帯水層の貯水量が激減しているためだ。土の保水能力は,私たち人類を含む地上の生物の未来に大きな影響を与える。
(3) Decompose and purify organic matter and chemical substances (decomposition and purification function)
土の中の生物が,動植物の遺体や動物の排泄物などの有機物を分解することは,前回お話しした。この有機物の分解で養分循環が生み出される。
各種の有害な有機性廃棄物なども,土の中での分解過程で浄化される。人工の化学物質,たとえば,石油成分やトリクロロエチレン,ポリ塩化ビフェニール(PCB)といった,かつて問題視されたいわゆる環境ホルモンの前駆物質である,有機塩素化合物を分解する微生物も土に生息する(内山,1999) 。微生物の汚染物質分解能力を利用して汚染物質を浄化し,環境を修復することをバイオレメディエーション(生物的修復)といい,安全な環境汚染修復対策として注目されている。
However, the function of decomposition and purification of organic matter is not a function of the soil itself but a function derived from the biological activity of the soil.
2. soil supports and preserves the global environment
The Earth's environment is maintained by the smooth circulation of heat, energy, water, chemicals, and other materials between the atmosphere, biosphere, hydrosphere, and geosphere that make up the Earth's surface. Most of the transfer of materials and energy between these spheres takes place through the soil, which is the contact surface (interface).
The rate of movement of matter and energy in the soil controls the speed of that movement. The physical, chemical, and biological properties and functions of soil can change the flow of water, heat, and materials from a rapid state to a gentle state, and in some cases, they can be stored in the soil to inhibit the flow itself. The ability of soil to soften the rate of movement of materials (buffering capacity) is a fundamental factor in ensuring the harmonious and smooth circulation of materials and energy on the earth.
Since the 20th century, human activities have expanded on an unprecedented scale, adversely affecting the global environment. Unprincipled deforestation to expand arable land, massive consumption of fossil fuels to increase productivity, and other human activities have caused the flow of materials and energy to exceed the control of the soil. The resulting changes in the global environment have had adverse effects such as global warming, depletion of the ozone layer, desertification and salinization of the soil, soil erosion, water pollution, and reduction of biodiversity.
3. soil functions are threatened by 10
かけがえのない土が,人間活動に起因してその生産機能を失ってしまう現象を土の劣化という。劣化した土地面積は,20世紀末で,地上の植生地の17%,20億haにも達している(国連環境計画,1997) 。
2015年に公表された世界土壌資源報告書(FAOと土に関する政府間パネル=ITPS,2015)によれば,土の機能に対して10の深刻な脅威があるという。その脅威を強さの順に並べると,①土壌侵食,②有機炭素の変化,③養分の不均衡,④塩類集積とナトリウム化,⑤土壌被覆(コンクリートやアスファルトなどで土を被覆して,透水性を阻害すること) ,⑥土の生物多様性の減少,⑦土壌汚染,⑧酸性化,⑨土壌圧密,⑩湛水(とくに好気的状態から湛水状態に変わる場合)である。
土の機能が,様々な脅威や人間活動で奪われている。しかし,農家の適切な管理が農地の土の機能を維持しているのもまた,事実である(図2) 。

−第4章− 土のでき方と地球上での役割
第23回 原始地球に土はなかった
−こうして地球に土が誕生した
令和5 (2023) 年 7月号 (第752号)
All this time we have been talking about soil. This was based on the assumption that soil is all around us. However, has soil been on the earth since the birth of the earth 4.6 billion years ago? The answer is no.
In this issue, I would like to return to the primitive earth and reflect on the story of how soil came to be on the earth and how it supports life.
1. the earth before the appearance of soil
今,私達の足もとには土がある。しかし,都会のアスファルト舗装された中で生活する人には,土があることさえ忘れているかも知れない。それでも郊外に出て広がる農地や野山を眺めると,そこに土のある風景に巡り会える。それが日常である。その土なのだが,現在の私たちが考える土に似た物質(初期の土)がこの地球上に登場したのは,今から6億年ほど前と考えられている。そして,私達がイメージする土が登場したのは3億年ほど前である。地球上に土が誕生するには,以下に述べるようなドラマがあった(図1) 。
4.6 billion years ago, asteroids in the universe collided with each other, accumulating energy and gradually expanding to form the Earth. Immediately after its birth, the earth was in a state of bare magma, with temperatures reaching over 1,000°C. Later, as asteroid impacts decreased, the magma cooled over time. Later, as the number of asteroid impacts decreased, the magma cooled with the passage of time. When the surface temperature reached about 300°C, water vapor fell to the ground in torrential rain. This became the ocean. This was about 4 billion years ago. This ocean was hot and highly acidic, with hydrochloric acid as its main ingredient. This is because the rain dissolved the gases emitted from the magma.
At that time, the atmosphere was 97% carbon dioxide (carbon dioxide gas). Carbon dioxide could not dissolve into the strongly acidic ocean. However, as the dissolved components from the rocks gradually neutralized the oceans, carbon dioxide gradually dissolved into the oceans, further neutralizing the acidic oceans.
Life was first found in the sea. This is because the sun's ultraviolet rays are harmful to living organisms, and the only place where they could avoid these rays was in the sea. This is thought to have occurred approximately 3.8 billion years ago. It seems that life began with bacteria that could grow in anaerobic conditions, that is, in the absence of oxygen.
2. birth of life and formation of the ozone layer
20億年前まで時がすすむと,海水中の生命のなかに光合成の機能を身につける細菌が現れた。それがシアノバクテリア(ラン藻,かつては植物の仲間と分類されていたが,現在は細菌として扱われる)である。シアノバクテリアは,海水中の無機物(ミネラル)と炭酸カルシウムとを反応させて層状に堆積するストロマトライトと呼ばれる岩石状物質をつくりだした。その表面に自身も生き残り,光合成をおこなって酸素の放出を続けた。それによって,大気に酸素が徐々に含まれるようになった。そして今からおよそ6億年前,大気の酸素濃度が2%にまで増えると,オゾン層が大気中にできはじめた(図1) 。
The ozone layer blocked ultraviolet radiation from the sun. This blockage of ultraviolet radiation, which is harmful to living organisms, has allowed marine life to come ashore. The first organisms to come ashore were lichens. Lichens are organisms in which algae (cyanobacteria, green algae, etc.) coexist inside a structure made of fungal mycelium. The photosynthetic products produced by the algae are used by the fungi to support their life, allowing them to grow even under conditions with limited nutrient sources.
3. birth of soil
その地衣類が陸上の岩石にとりつき,岩石を変質させていった。同時に,地衣類自身が死ぬと,その遺体が有機物となって次世代の栄養分になるだけでなく,変質した岩石にも混じり合って,地球上に初期の土ができはじめた。およそ6億年前のことである。その後,この作用が繰り返されることで,ゆっくりと土がつくられていった。
こうして,およそ3億年前には,地球上に土ができていたと考えられている。それは,当時の陸上には封印木,蘆木などの巨大なシダ植物の森林ができ,両生類,昆虫類が出現したことからうかがい知ることができる。それらが現在の化石燃料を提供している(図1) 。
It took a long journey for soil to be formed by the organisms that came to life on the earth. That path was completed at that time, and it has not ended, but continues to the present day. At first glance, the soil appears to be immovable. However, as an extension of this enormous change over time, the soil is still changing to harmonize with its environment.
4. the earth's "skin" supports terrestrial life
The earth is in the exquisite position of being the third closest to the sun. Too close to the sun and water evaporates and disappears. If it is farther from the sun, it will freeze. The exquisite position means that there was enough distance between the sun and the earth for water to exist stably. This water and the oxygen in the atmosphere made it possible for life to survive on Earth.
地球は半径およそ6400kmのほぼ球体である(図2) 。この球体の中心部が核で,その外側がマントル,そしてマントルの外側に地殻がある。陸地は,地球の表面積の30%程度にすぎない。陸地の地表から30〜40kmの厚さで覆われる部分が大陸地殻である。土は大陸地殻の表面のほんの数cmからせいぜい数m,全地球を平均すると18cmの厚みしかないという(陽,1994) 。この厚みは,地球の半径の1000万分の1にすぎない。人の皮膚は平均すると2mm,身長2mの人の1000分の1である。つまり土は,地球規模でみると人の皮膚よりもさらに薄い,かすかな皮膚にすぎない。地球を図2のように表現すると,18cmの厚みは,図に示した円の外周線の太さでも厚すぎ,図示すらできないほどの薄さである。
Plants grow in the soil, which is a faint skin of the earth, and microorganisms and animals live on it, and much of our food is produced from the soil. The soil, which is only a small part of the earth as a whole, supports the life of all terrestrial life on earth.
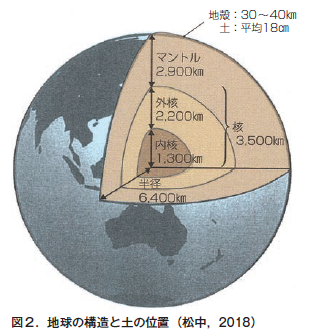
−第4章− 土のでき方と地球上での役割
第24回 土は環境の産物である
−風化と生物の作用が岩石から土をつくる
令和5 (2023) 年 8/9月合併号 (第753号)
About 600 million years ago, 4 billion years after the birth of the earth 4.6 billion years ago, something like soil (early soil) was formed, and about 300 million years ago, the soil we imagine was formed on the earth. In the last issue, I told you the story of the formation of soil. This time, I would like to talk about how soil is created by the environment.
1. change in perception of soil
土は,これまでどのように認識されてきたのだろうか。19世紀まで,土は岩石が風化した地殻の表層にあるやわらかい物体,というくらいにしか考えられていなかった。この考え方を,土は環境によってつくられるという見方に転換させたのが,ロシアの若き地質学者で,後に土壌学の祖といわれるドクチャーエフ(1846−1903)である。
He believed that soil is formed by the interaction of various factors such as rocks, which are the raw materials of soil, climate, plants and animals, and topography, and that the formed soil changes with time. He also argued that soil, like animals and plants, is one of the components of nature and that soil is a product of the environment. Let us look at this idea in detail below.
2.土をつくる二つの要因風化と生物の作用
わが国では,火山灰を原料とする土 (黒ボク土)が広く分布している。しかし,世界的にみると黒ボク土は例外的な土で,一般的な土の原料は岩石である。この原料となる岩石を母岩という。その母岩から土ができるには,岩石が細かく砕かれる作用(風化)と,その砕かれた岩石に生物が働きかけて土をつくりあげていく作用の二つが必要である(図1) 。
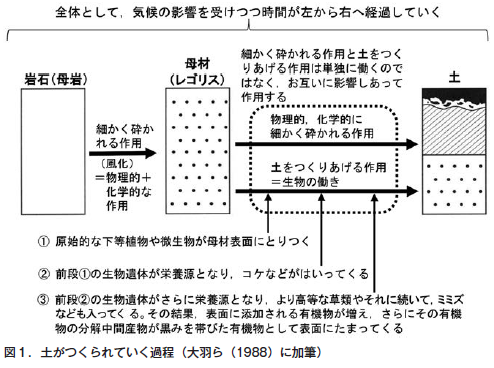
土ができるためにまず必要なことは,風化によって母岩が細かく砕かれた物質(これを土の母材という)の表面に,先月号述べた地衣類や微生物たちがとりつくことである。そしてそれらがそこでの生活をまっとうして死ぬと,その遺体は別の微生物によって分解される。その分解産物が植物の養分になる。この養分が蓄積すると,植物が生活できる環境が備わり,そこへより高等な植物であるコケ類やイネ科の草などが侵入する。コケや草が入ってくると,これらの遺体が微生物に分解されて養分がさらに増える。そうすると,土の動物(ミミズなど)が住めるようになる。土の動物たちは,植物遺体の分解産物として母材に蓄積していた有機物をエサにして生き,死んでいく。その結果,母材の表面は養分がさらに豊かになっていく。こうして,より高度な植物が生活できるようになる。そしてその高等植物が枯れて遺体になるということがくり返されると,徐々に母材の表面に,有機物と母材とが混じり合った黒い色を帯びた土の表層ができあがっていく。
The action that creates soil in this way is the result of the action of living organisms on the parent material. No matter how much a rock is weathered and crushed into small pieces, soil cannot be created without the presence and action of living organisms.
3. the function of soil-building organisms is affected by the environment
The workings of the organisms that make up the soil are closely linked to the environment. This is because the types of organisms and their activity are greatly influenced by the climate.
In cold regions at high latitudes, the amount of organic matter added to the soil is low because plant growth is poor. However, because of the cold weather, decomposition of organic matter by microorganisms does not progress, and organic matter accumulates, resulting in dark soil with a darker color, as indicated by the organic matter. On the other hand, in low-latitude tropical rainforest areas, the amount of organic matter added to the soil is much higher than in colder regions because of vigorous plant growth. However, because of the higher temperatures, the decomposition of organic matter by microorganisms is faster, and organic matter is less likely to accumulate in the soil. Therefore, the soil in this region does not darken, and a reddish-brown color is produced.
Soil is formed and changes within the limits of given environmental conditions, not at will. This is why Dochkiaev describes soil as "a product of the environment," and asserts that "soil is not some mechanical, accidental, lifeless mixture; on the contrary, it is a natural history formation (a historical natural body) determined and governed by independent and fixed laws. In other words, given certain environmental conditions, the same soil will be produced if the base material is the same. However, even if the base material is the same, if the environmental conditions are different, the resulting soil will be different. The environment creates the soil.
改めて世界の土を眺めると(図2) ,土は地球の緯度に沿って帯状に分布しているように見える。緯度に応じて気候が大きく変化し,その変化に対応した生物の異なる働きで土ができるからである。巨視的にみれば,ドクチャーエフの指摘どおり,「土は環境の産物である」ことが実感できる。
The moon is devoid of life. Therefore, the biological processes necessary to create soil do not work. Therefore, there are rocks on the moon, but no soil. Because the earth is at a perfect distance from the sun, the atmosphere and water can exist, and organisms were born. Thanks to the work of these organisms, soil was created and agriculture began.
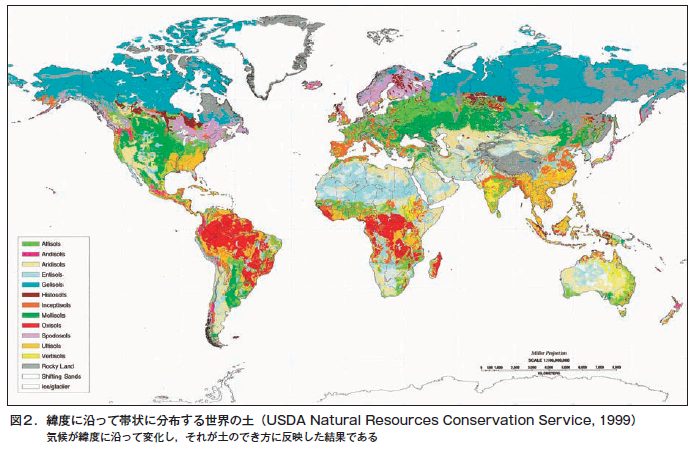
−第5章− 農業に起因する環境問題
第25回 農業が環境破壊の始まり
−人間活動と環境との関わり−
令和5 (2023) 年 10月号 (第754号)
Soil is created in a given environment through the work of the living organisms present there. This is the idea of the Russian soil scientist Dokuchaev, which I mentioned last month. As if to prove the correctness of this idea, from a macroscopic viewpoint, the world's soil is distributed along latitudinal zones corresponding to climatic changes. This is because climatic conditions have a great influence on the environment. We can truly feel that soil is a product of the environment.
This month, we will consider the relationship between the soil and the environment in terms of human activities, especially in terms of food production.
1. the beginning of agriculture
土を利用して,自分たちの食べものを確保しようとしたのは,およそ4万年前,旧石器時代のクロマニョン人である(タッジ,2002) 。彼らは,狩猟採集で食べものを得ていたネアンデルタール人とはちがう方法で,食べものを手に入れた。動物の捕獲も行き当たりばったりではなく,動物の習性からどこで待ち伏せすればよいかを考えた。そして仲間で役割を分担し,動物の群れを追跡し,囲い込んで捕まえるという新しい狩猟方法を生み出した。また,変化に富む新しい道具も作った。同時に,彼らの食べものとなる植物を保護し,育てることもおこなった。それは,農業の始まりの前段階ともいえる出来事である。そして1万年ほど前,今でいう農業を取り入れた生活が始まったと考えられている(ポンティング,1994) 。
この農業の始まりは,人が自然に働きかけて土地を切り拓き,そこの土で食べものを栽培するという積極的な行為だった。それは人の暮らしを変化させた。もちろん,当時は自然環境のほうが圧倒的な力で人類を支配していたはずだ。しかし,人類が自然に挑戦した最初の出来事だった。与えられている自然環境に人の手を加えるという意味では,環境破壊のはじまりとも理解できる(図1) 。人間活動が自然環境の力より弱い段階では,それは大きな問題とならなかった。

2. the center of human productive activity changed with the Industrial Revolution
However, when human activities become so active that they alter the natural environment, the altered natural environment cannot be restored. It was the Industrial Revolution that reversed the power relationship between human productive activities and nature.
18世紀後半から19世紀の前半にかけて,イギリスで起こった産業革命は人の暮らしを一変させた。石炭を使ったエネルギー転換で機械化することを知った当時の人たちは,それを利用して経済活動を大きく発展させた。それまで,農業が人間の生産活動の中心であった。しかし,産業革命はそれを工業に転換させてしまった。
Around the same time as the Industrial Revolution, the hyper-intensive Norfolk farming system was established. This led to a dramatic increase in agricultural productivity. This was a factor in increasing the rural population. Ironically, the increased rural population was sent to the cities as factory workers, which were needed in the Industrial Revolution. This led to a rapid increase in the urban population. The total population of pre-industrial England in 1801 was 8.32 million. The urban population was 3.66 million, or 44% of the total population. Eighty years later, in 1881, after the Industrial Revolution, the total population had tripled to 25.91 million, and the urban population had quintupled to 17.28 million, 67% of the total population.
3. commercialization of food has changed the value of food
Both to increase the population and to provide food for the increased population, it is necessary to increase the production of food. The increase in the production of food was demanded in the rural areas. This situation further led to the enclosure of farmland. At the same time, farm management, in which capitalists who leased large tracts of land from landlords hired peasants as wage laborers to turn the food they produced into a commodity and make a profit from its sale, emerged and expanded. Thus, with the Industrial Revolution, communal subsistence management disappeared from English farming villages. This transformation of rural society that occurred with the Industrial Revolution in England in the latter half of the 18th century is called the Agricultural Revolution.
この変化はその後の経済のあり方を大きく変えた。それまでの自給自足的農業経営では,食べものは空腹を満たすことで人に役立つ(これを使用価値という)ことが重要な要素であった。しかし,資本家が経営する農場では,消費者である賃金労働者へ商品としての食べものを生産することが目的となる。このため,生産した食べものをいくらで売って,いくら利益を得るか(これを交換価値という)が重要になる。つまり生産した食べものの価値が,使用価値から交換価値へ転換していった。そうなると,それまでの農業が生産性の維持のために,大気,水,土といった人類共通の資源の保全にかけていた費用(コスト)は,利益を増やすという意味からみて,短期的にはマイナスの費用になる。この費用を自然環境に転嫁し,資源を無料で消費する農業のほうが利益を増やせるという考え方が,しだいに広まった。
4. criticizing plundering agriculture without nutrient cycle, Liebig
It was Liebig, who lived during the Industrial Revolution, who strongly criticized this waste of resources, especially agriculture, which only takes crop nutrients from the soil and does not replenish them, thereby reducing the fertility of the soil.
産業革命後のイギリスでは,農村と都市で分業が進んだ。主食の穀物は農村で生産し,その穀物を消費するのがロンドンなどの都市労働者という時代になっていった。農村の農地の土から吸収された作物養分は,収穫物である穀物に含まれたまま都市に運ばれる。都市労働者が食べた穀物に含まれる作物養分の一部は労働者の排泄物に移行し,その排泄物は夜,窓辺から街路に投げ捨てられた。当時の都市は下水道が整備されていなかったため,それらは雨に流され,ロンドンでは直接テムズ川に運ばれた。このため,テムズ川は強烈な悪臭にまみれ,不衛生きわまりなかった。こうして,農村の土にあった作物養分は,元の農地の土へ戻れなくなった。リービヒは,そうした土の養分を消費するだけの「略奪農業」では安定した食料供給ができないと強く批判し,農業の持続性に最も重要なことは養分循環であることを強調した。
そのリービヒが養分循環の理想としたのが日本の農業だった。江戸時代の17世紀以降には,当時の世界的大都市江戸の消費者のし尿が,彼らに食料を提供する周辺農村の農地に還元される経路がしっかりと確立されていたからである(リービヒ・吉田訳,2007) 。
産業革命以降,人々の暮らしは豊かで便利になり,人間活動がますます大きくなっていった(図2) 。自然から預かった社会共有の財産であるはずの土から,資源を勝手気ままに利用して使い捨てるという利己的な経済活動が大きな流れとなってしまった。現在では,そうしたことで,農業自身が環境汚染源となってしまうという問題まで発生している。具体的にどのような問題があるのか,それを来月号以降で見てみようと思う。

−第5章− 農業に起因する環境問題
第26回 農業と環境問題−その1
わが国の窒素循環の問題点
令和5 (2023) 年 11月号 (第755号)
The beginning of agriculture was the first human challenge to nature. In the sense of adding human intervention to the natural environment, it can be said to be the beginning of environmental destruction. Later, after the Industrial Revolution, human activities became so active that they altered the natural environment. Agriculture is no exception.
In many countries today, environmental pollution originating from agriculture is a problem. When the amount of nutrients applied to agricultural land exceeds the nutrient holding capacity of the soil, the excess nutrients flow into the groundwater, rivers, and air in the surrounding environment, causing environmental pollution. This month, we will consider the relationship between agriculture and environmental pollution.
The first issue is unique to our country, which has a low food self-sufficiency rate.
Changes in Japan's food self-sufficiency rate
In 1960, the Japanese government made two major cabinet decisions. The first was to accelerate economic growth through the Income Doubling Plan, and the second was to enact the Basic Agricultural Law. In 1960, Japan's food self-sufficiency rate (hereafter referred to as the calorie-based total calorific value of food supply) was 79%. Since then, however, Japan's food self-sufficiency rate has continued to decline, stagnating at 38 to 39% since 2010. The target for food self-sufficiency is 45% by 2030. Achievement of this goal is in jeopardy.
過去60年間にわたってわが国の食料自給率を低下させた主な要因は,食料を国内でまかなうという基本姿勢が維持されなかったこと,さらに食料の消費形態が変化したことにある。
例えば,1965年度と2021年度の56年間で比較すると,1人1日当たりの各品目の供給熱量を合計した総供給熱量は,1965年度が2,459kcalで,2021年度はやや少なくなって2,256kcalだった(図1) 。ところが,この56年間で米の消費は大きく減少し(供給熱量の減少),その減少は畜産物(肉類,鶏卵,乳製品など)と油脂類(大豆油,菜種油など)の消費拡大(供給熱量の増加)で相殺されている(図1) 。さらに,総供給熱量に占める割合は56年間で大きな変化がないにもかかわらず,供給熱量の自給率が大きく低下した品目として果実,魚介類,小麦,大豆,野菜,そして「その他」に区分される品目がある(図1) 。
こうした食料の消費形態の変化や品目別の自給率の低下は,国民の食傾向がごはんと魚という「和風」から,パンやパスタ,乳肉製品という「洋風」へ変化したことに起因する。ただし,この食料の消費形態の変化は,それが国民の単なる嗜好の変化や,所得の増加といった「成り行き」の結果とは考えにくい。むしろ,第二次世界大戦後のアメリカの余剰農産物輸出戦略に呼応した,わが国の政策的誘導の結果と見るべきだろう(柏,2012) 。
Food production, consumption and nitrogen cycle in Japan
食料自給率の低さは,いいかえると外国の土にあった養分が作物に吸収され,それが含まれたまま輸入食料となって多量にわが国に持ち込まれていることを意味する。なかでも窒素(N)は,次回で述べるように,環境に悪影響を与えやすい。そのわが国でのN循環量が,自給率の低下によってどう変化したかを,データが報告されている1961年と2005年で比較してみる(図2) 。
(1) N circulating volume in 1961
食料自給率が78%だった1961年,輸入されたNは,作物から14万トン,プロテインミールから2万トンで,年間わずかに16万トンだった。一方,農耕地に供給されるNは化学肥料由来(年間63万トン)が最も多く,家畜のふん尿由来のN(同16万トン)は,生物的N固定で土にはいる量(同22万トン)より少なかった。飼養されている家畜の頭数が少なかったからである。
最終的に1年間で地下水や河川へ流出したNは,農耕地からの55万トンと,人間の排泄物(下水処理前の値)からの38万トンの合計93万トンだった。このほか大気に揮散したN(アンモニアガスとして)は,化学肥料や家畜ふん尿由来の合計で2万トンだった。
2)2005年のN循環量
In contrast, in 2005, the population was 128 million, 35% more than in 1961. The food self-sufficiency rate fell to 40%, resulting in a large increase in total imported N, 5.6 times that of 1961, to 890,000 tons (680,000 tons from crops, 140,000 tons from protein meal, and 70,000 tons from livestock products). Conversely, the amount of N entering agricultural land as biological N fixation, chemical fertilizers, and crop residues has decreased. This is because domestic food production has decreased.
Increased consumption of livestock products requires an increase in the number of livestock raised to support their production. The feed for the increased number of livestock is dependent on imports. Therefore, N contained in the imported feed is turned into livestock manure, which flows into agricultural lands. The amount of this inflow was 510,000 tons in 2005, 3.2 times the amount in 1961. Furthermore, the amount of N discharged from human excrement was also 1.8 times that of 1961. This is more than the population growth rate (35%), confirming the high protein content of food.
As a result, it was estimated that in 2005, the amount of N discharged from agricultural lands into groundwater and rivers increased to 840,000 tons per year, and the total of 670,000 tons discharged from human excrement into the environment was added, resulting in an annual discharge of 1.51 million tons into groundwater and rivers. This amount is 1.6 times that of 1961. The amount of N volatilized as ammonia gas from fertilizers and livestock manure into the atmosphere was 60,000 tons, three times the 1961 level.
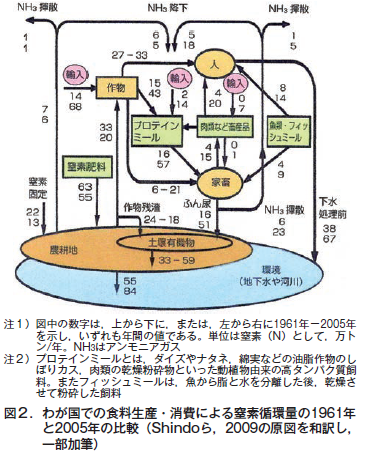
わが国の低い食料自給率と食料消費動向の変化は,海外から持ち込むN量を大きく増やした。それがわが国の地下水,河川などの水質汚濁や,大気環境の悪化を招く可能性は大きい(Shindoら,2009) 。まさに低自給率ゆえの問題である。
次回は,この環境に流出するNが具体的にどのような環境汚染源となっているかを考える。
−第5章− 農業に起因する環境問題
第27回 農業と環境問題−その2
農地由来の窒素による水質汚濁
令和5 (2023) 年 12月号 (第756号)
前回述べたように,わが国の食料自給率は低いため,輸入される食料や家畜飼料などに含まれる窒素(N)などの作物養分が,海外から多量に持ち込まれている。畜産農家に飼料や肥料の形で多量の養分が持ち込まれると,その農場での土−飼料−家畜を巡る養分循環が破綻し,養分が周辺環境へ流出する。養分の中でも,とくにNは環境への悪影響が大きい。耕種農家でも,外部から農地に持ち込まれるNが土の保持容量以上になると,Nが周辺環境に流出する。流出したNが,河川や地下水へ到達すると水質汚濁を招き,大気に到達すると大気汚染となって環境を汚染する。
In this issue, we will consider water pollution among the environmental pollution caused by N discharged from agricultural land. Air pollution will be discussed in the next issue.
1. eutrophication caused by water pollution
Sources of substances that adversely affect rivers, lakes, marshes, and other bodies of water through groundwater and surface runoff can be broadly classified into two categories: point sources (specific sources), which can be identified, and areal sources (non-specific sources), which cannot. Point sources (specific sources) and non-specific sources (non-specific sources). In addition to N, other substances such as phosphorus (P) and organic substances are discharged from these sources and cause water pollution.
Nは通常アンモニア態N(NH4⁺−N)の形態で農地に与えられる。与えられたNH4⁺−Nは,土の微生物によって硝化作用を受け,負荷電イオンの硝酸態N(NO3⁻−N)に変化する。Pも通常は,負荷電のリン酸二水素イオン(H2PO4⁻)として土に存在する。つまりNやPの形態はいずれも負荷電のイオンであるため,土が持つ負荷電と反発しあって土に保持されにくい。それゆえ,NやPは河川や地下水に流出しやすく,環境汚染物質になりやすい。
それらが湖沼や河川に流入することで,その濃度が高まり養分が富化されていく(これを富栄養化という) 。富栄養化すると植物プランクトンや浮遊性ラン藻が異常発生して,緑のペンキを流したように水面を覆いつくしてしまうことがある(図1) 。これが水質汚濁の終着点である。

2. sources of environmental pollutants
1)点源汚染
(1) Point source contamination
典型的な点源汚染としては,以下のような例がある。すなわち,牛舎,豚舎,鶏舎などの周辺や工場,下水処理場などの近くを通過した河川のN濃度の上昇,あるいは,素掘りふん尿だめ(ラグーン)に貯留したふん尿混合物由来Nによる地下水の汚濁,さらに,放牧家畜が水飲み場として小河川を利用する時,河川に直接ふん尿を排泄することなどである。畜産関連施設近くの井戸水のNO3−−N濃度が飲用基準(1リットル当たり10mg)を上回るという各地の事例は,点源汚染の実例である。
2)面源汚染
農地は,森林や市街地などとともに広がりのある土地,すなわち面源として扱われる。面源による汚染が面源汚染である。この汚染では発生源が特定されないため,環境汚染物質がどこから発生し,地下水や河川,湖沼へどの程度流出しているかを定量的に把握するのが難しい。しかし,単位集水域面積当たりの家畜飼養頭数が多くなると,それにともなってN流出量も増加するため,河川水のN濃度が高まる(図2) 。つまり面源汚染では,環境へ汚染物質の流出量が増加すれば汚染は確実にすすむ。
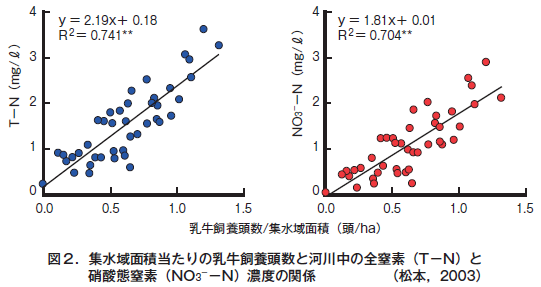
Water Pollution Prevention Measures
1)点源汚染対策
Point source pollution in livestock farms often occurs when manure overflows from storage facilities and leaks into the environment because the size of the storage facility does not meet the appropriate capacity for the number of animals being kept. To prevent this, manure storage facilities have been regulated by the "Law Concerning the Proper Management and Promotion of Utilization of Livestock Manure" since 1999, and penalties have been imposed on facilities that violate the law.
2)面源汚染対策−①許容限界N量の設定
面源汚染を防ぐには,地下浸透水のNO3−−N濃度の監視が重要である。NO3−−N濃度は,農地に与えるNと作物が吸収するNの収支結果と,土を浸透する水の量によって決定される。したがって,NO3−−Nの地下浸透による水質汚濁を防ぐには,まず農地に与えるN量を,自然の自浄力によって汚染物質による環境への悪影響が生じない環境の収容力(これを環境容量という)の範囲に抑えておくことが重要である。この他,作物のN吸収が旺盛でない時期にNを農地に与えることも避けるべきである。
このような考え方に基づいて,EU(ヨーロッパ連合)では家畜ふん尿由来のN量は,農地に対して170kg/haまでを許容限界量として法的に規制している。わが国ではこのような面源汚染の規制がない。そのため,ふん尿貯留施設に法的規制をかけて点源汚染を防止しても,農地に与えるN量を規制する限界量がなく,面源汚染防止が徹底されない。その結果,単位面積当たりの家畜飼養頭羽数が多い9県(群馬,神奈川,愛知,徳島,香川,長崎,宮崎,鹿児島,沖縄)では,地下水に流出するN濃度の推定値が,いずれも飲用基準を超えると指摘されている(寳示戸ら,2003) 。
3)面源汚染対策−②地形連鎖の利用や緩衝帯の設置
具体的な面源汚染防止対策として,例えば茶園−畑−水田−湿地−河川というように,高位置から低位置へ続く地形連鎖の利用が有効である。高位置の農地から地下浸透したNO3−−Nは,低位置の農地の作物で再利用され,最終的に水田や湿地などの還元条件(酸素が不足した状態)で環境に無害な窒素ガス(N2)に変換されて大気に排出されるため,面源汚染の防止につながる。
農地から表面流去水として水域へ流出する場合は,流出したNO3−−Nなどの環境汚染物質が河川や湖沼に到達するまでに,自然浄化を受ける機会を多くすることが汚染防止対策となる。そのために,河川のそばに湿地や林地を設けて河川への流入の緩衝帯として利用すると,自然浄化がすすみNO3−−N濃度が低下する。
浄化の程度は発生源と河川の間隔が,裸地状態よりも草地のように作物が栽植された状態のほうが大きい。この目的で設置される緩衝帯の必要幅は,点源汚染対策で数〜数十m,面源汚染で数十mとされている。ただし,この緩衝帯の効果は土地条件で大きく変化するため,緩衝帯の必要幅についての具体的な基準は示されていない。
−第5章− 農業に起因する環境問題
第28回 農業と環境問題−その3
農地由来の窒素による大気汚染−アンモニア揮散
令和6 (2024) 年 1月号 (第757号)
Livestock production in Japan has developed through dependence on imported concentrate feed. This has been the result of policy inducements since the end of World War II. This trend remains unchanged to this day, and the feed self-sufficiency rate is extremely low at 26%. Dependence on imported feed makes it possible to run a livestock business even if the farmland to produce feed for livestock is small. Thus, livestock production detached from the land increased the number of head of cattle raised, regardless of the area of land used for management. However, this means that instead of using the manure excreted daily by livestock as an important source of nutrients, it is likely to become a source of environmental pollution by dumping large amounts of manure on small farmlands.
今月は,先月に続き農地から環境に流出する窒素(N) ,とくに家畜ふん尿由来Nのアンモニア揮散による環境問題を考える。
1. negative environmental impact of volatilized ammonia
家畜ふん尿に含まれるアンモニア態窒素(NH4−N)は,草地表面に与えられた時のように,大気に触れることでアンモニアガス(NH3)になって大気中に揮散する。
NH3揮散は,与えた肥料養分としてのNの損失だけでなく,揮散したNH3が大気中の硫黄酸化物や窒素酸化物,塩化物などと結合し,硫酸や硝酸,塩酸を含む雨となって降下するため,より強い酸性雨の発生源になる。こうして降下したNH4−Nは,樹木やその他の植物の生育をかく乱し,土に浸透して硝酸化成作用を受けることで土のpHの低下と,それに伴う土の養分バランスを悪化させるなど,環境に大きな悪影響を与える。
なお,化学肥料を土の表面に与えても,その土が特別なアルカリ性でないかぎり,NH3揮散は少なく,尿素でごくわずかに検出できる程度である。また,家畜ふん尿が2cm程度のわずかな厚みでも土に覆われると,NH3揮散はほとんど発生しない(Matsunakaら,2008) 。
2.草地表面に与えられた家畜ふん尿からのNH3揮散とそれに関わる要因
草地表面に与えられた家畜ふん尿からの単位時間当たりNNH3−N揮散量(NH3−N揮散速度)のピークは,おおむね与えられてから数時間以内に現れ,NH3揮散は数日以内で終了する(図1) 。
与えられたNH4−N量に対する揮散したNH3−N量の割合をアンモニア揮散率(以下,NH3揮散率と記す)という。乳牛スラリー(乳牛が排泄したふんと尿,それにオガクズのような敷料が加わった混合物)を草地表面に与えた場合,アンモニア揮散率はスラリーの与えた量が60t/haまでなら30%程度である(Matsunakaら,2008) 。
このNH3揮散率は,スラリーのpHや乾物率が高く,また,与えた時の気温が高いほど高まる。しかし土が乾燥していると,スラリーのNH4−Nが土の中に浸入しやすくなるため揮散率は低下する。これらの要因のうち,気温がNH3揮散に最も大きな影響を与える(Matsunakaら,2002) 。
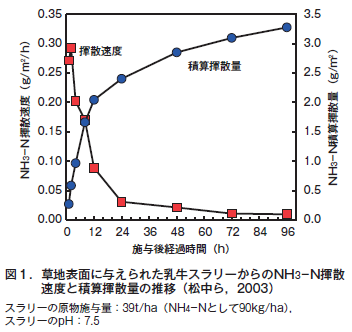
3.草地表面に与えられた家畜ふん尿からのNH3揮散を抑制する対策
草地や土の表面に与えられた家畜ふん尿からのNH3揮散を完全に防ぐことは,与えた後に多量の降雨がないかぎり事実上不可能である。しかし,ふん尿の与え方を工夫し,与えたふん尿を大気に触れにくくすれば,揮散率の低下につながる。
例えば,乳牛スラリーを草地の表面に与える時に利用する方法として,衝突板方式(スプラッシュプレート,図2−a) ,帯状施与法(バンドスプレッド,図2−b) ,浅層注入法(シャロウインジェクション,図2−c)などがある。このうち,わが国で一般的に利用されているのが衝突板方式である。

この方式は他の与え方に比べて,スラリーが空気に触れやすいため,NH3揮散率が最も高い(図3) 。この与え方に比較すると,浅層注入法なら揮散損失を77%削減でき,帯状施与法は揮散損失を36%削減するだけでなく,ふん尿散布後の悪臭強度を低下させる効果も大きい(図4) 。
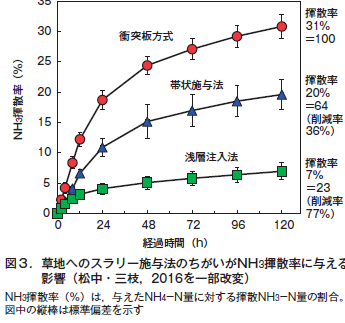
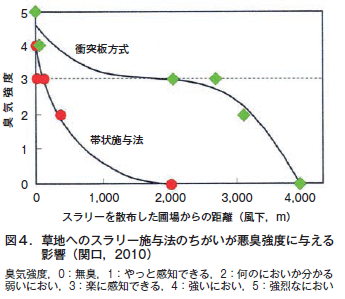
農業による環境汚染の抑制に積極的に取り組むEU(ヨーロッパ連合)では,先月述べたように,ふん尿由来Nの農地への投入量を最大170kg/haに法的規制している。それだけでなく,環境への悪影響が大きいNH3揮散を抑制するために,揮散率が高く,悪臭強度が強い衝突板方式による乳牛スラリー散布を禁止している。わが国ではこのような強い規制がないまま,家畜ふん尿による環境汚染問題が継続している。
−第5章− 農業に起因する環境問題
第29回 農業と環境問題−その4
農地由来の窒素による大気汚染−一酸化二窒素排出
令和6 (2024) 年 2/3月合併号 (第758号)
今回も,前回のアンモニア揮散に続き,農地に由来する窒素(N)による大気汚染の話題で,温室効果ガスの主要なものの一つ,一酸化二窒素(N2O,亜酸化窒素ともいう)を取り上げる。
なお,N2O以外の温室効果ガスについてや,地球規模からみた温室効果ガスと農業との関わりは,次回,総合的に考えることにする。
1. N2O generation (direct emission) and soil moisture conditions
N2Oの温室効果は,二酸化炭素(CO2)の298倍も強力である。また,成層圏オゾン層の破壊にも関与しており,環境への悪影響が大きい。
農地でN2Oが発生するのは,微生物の働きが関わる二つの経路がある。一つは,家畜排泄物などから生産される有機質肥料や化学肥料の形態で,農地に与えられたアンモニア態窒素(NH4−N)が酸素のある条件(酸化的条件)で,硝酸態窒素(NO3−N)に変化する時(この変化を硝酸化成という)の副生成物として発生する経路である。もう一つは,硝酸化成でできたNO3−Nが,酸素不足の条件(還元的条件)に置かれて,窒素ガス(N2)へ形態変化する(この変化を脱窒という)時の中間産物として発生する経路である。
土の中が酸化的条件であるか,還元的条件であるかは,土の中のすべての隙間(全孔隙)が水分でどの程度埋め尽くされているか(これを水分飽和度(略称WFPS=Water Filled Pore Space)という)で決まる。土の水分飽和度が60%(硝酸化成の微生物活動にはほどよい水分状態)から70%(土がやや湿った状態)の範囲で,硝酸化成や脱窒の二つの作用が進行するため,N2Oの発生が多くなって土から大気に排出される(図1) 。水分飽和度が80%を上回る湿潤な状態では,還元的条件が強まり脱窒によって,主にN2が生成して排出され,N2Oの排出は少ない。逆に,水分飽和度が50%より低い場合は,乾燥状態となり硝酸化成が中心で,主に一酸化窒素(NO)が排出される。
N2O排出に好適な水分飽和度60〜70%とは,水分状態が作物生産に比較的良好な状態と重なる。したがって,農地へのN施与量が適正量であったとしても,N2Oの発生を完全に抑止するのは難しい。また,地温が高まると微生物活性が高まってN2Oの発生量も多くなる。
このように土の中で発生したN2Oがそのまま大気に排出されることを直接排出という。
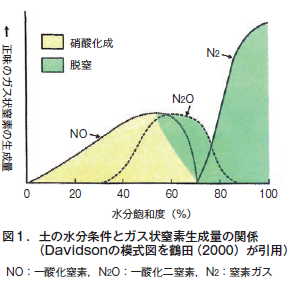
2.水に溶けたN2Oからの排出−間接排出
農地で発生したN2Oの一部は,土の中の水分(土壌溶液)や地下水に溶け込み,過飽和の状態で溶存している。この過飽和でN2Oを含む溶液が,暗渠排水や湧水,河川水として大気に開放されると,N2Oは過飽和状態が解除されるため,大気に排出されていく。さらに,前回お話ししたアンモニア揮散によって大気へ出ていったNH4−Nが,降雨に溶け込んで土に戻ってくると,土の中で硝酸化成を受け,その副生成物でN2Oが発生する。
Such emissions are known as indirect emissions and, like direct emissions, are not negligible.
3. N2O emissions in Japan and their relation to agriculture
2021年のわが国で排出されたN2Oの総量は,CO2に換算して19.9Mt(メガトン=百万トン)だった (日本国温室効果ガスインベントリ報告書,2023年) 。これは,1990年に比較し40%も削減されている(図2) 。このうち,農業分野から排出されたN2O量はCO2換算で9.6Mt,総排出量の48%を占める大きな排出源である。しかも工業プロセス及び製品の使用の分野では,1990年から2021年に排出抑制が大きく成功したのに対して,農業分野の抑制はわずかにすぎない(図2) 。
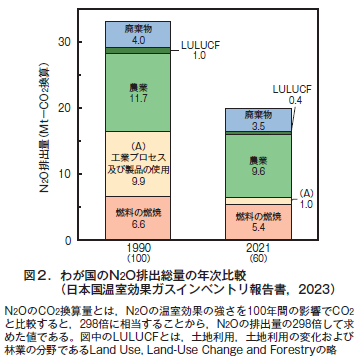
農業に由来するN2Oは,主に家畜排泄物の管理からと農地の土から排出される。すなわち,家畜排泄物を管理している時に,排泄物中で硝酸化成や脱窒が発生し,N2Oが直接排出される場合と,管理過程でのアンモニア揮散を起点とする間接排出である。農地の土に由来するのは,化学肥料や有機質肥料が農地に与えられた時,これら資材に含まれるNが,農地の土の中で硝酸化成や脱窒の作用を受け,N2Oが直接排出される場合と,生成したNO3−Nが地下水などに溶存した後,間接排出される場合である。さらに,農作物残渣の燃焼(野焼き)でも排出される。しかし,その量は極めてわずかである(図3) 。
わが国の農業分野からのN2O排出量(CO2換算量) は,1990年に11.7Mtあったのに対して,2021年は排出量が28%削減されて9.6Mtに減少した(図3) 。この削減は,農地の土から直接排出するN2O量の減少効果の影響が大きい。しかしそれは,この期間の国内の農地面積が大きく減少したため,農地へ施与される化学肥料や有機質肥料の総量も減少したという消極的な結果である(日本国温室効果ガスインベントリ報告書,2023年) 。
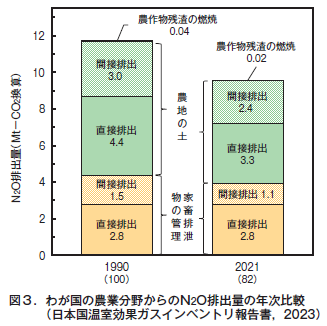
4. measures to control N2O emissions from agricultural land
日本国温室効果ガスインベントリ報告書(2023)によると,一部の地域では,環境保全型農業が推奨され,それが余剰Nによる地下水の水質汚濁を緩和し,その結果,N2Oの間接排出量の削減につながったという。農地を巡るN循環で余剰Nを発生させない環境保全型農業の実践は,N2O排出抑制効果が大きいと期待できるだろう。
この他にN2O排出抑制効果が期待される技術として,硝酸化成抑制剤の利用がある(Diら,2010) 。その抑制剤の一つジシアンジアミド (DCDと略)はすでに実用に供されている。DCDは硝酸化成に関わる微生物(細菌)のうち,硝酸化成の初期段階,すなわち,NH4−Nから二酸化窒素(NO2−N)への形態変化に関わるアンモニア酸化細菌の活性を低下させて硝酸化成を抑制し,N2Oの生成を減少させる働きがある。
There is uncertainty about the effectiveness of nitrification inhibitors in reducing N2O emissions in actual fields and grasslands. This is because the effect is influenced by environmental conditions such as soil moisture and soil temperature, which may or may not be apparent.
−第5章− 農業に起因する環境問題
第30回 農業と環境問題−その5
農業由来の温室効果ガスと地球温暖化
令和6 (2024) 年 4月号 (第759号)
Previously, we have discussed the negative effects of nitrogen from agricultural land on the environment in four articles. In particular, we have discussed that air pollution caused by nitrogen from agricultural land is related to the emission of dinitrogen monoxide, a greenhouse gas, and contributes to global warming. In this article, we will consider the relationship between global warming and agriculture, including other greenhouse gases.
1. greenhouse gases and their merits and demerits
地球上に生命が宿ったのは,水が存在できたことや,大気のおかげで地表面の気温が平均15℃くらいに維持されたことなどが大きな要因だろう。
この気温を維持する働きは,大気中の二酸化炭素(CO2)やメタン(CH4) ,一酸化二窒素(N2O,亜酸化窒素ともいう) ,それに人工の化学物質であるフロンガス類などの気体が担っている。これらの気体は,太陽の熱エネルギーの一部を吸収する性質を持つ。この性質を温室効果といい,温室効果をもつ気体を温室効果ガスという。気体の水,すなわち水蒸気も温室効果ガスの一つである。
人類は,化石燃料を燃焼させて動力を獲得して産業革命に成功し,大量生産,大量消費の時代をつくり出した。化石燃料の燃焼は,大気へ温室効果ガスのCO2を大量に排出する。その結果,大気中のCO2濃度は2023年に419ppm(暫定値)となった(Friedlingsteinら,2023) 。これは,産業革命前(1750年)の278ppmの1.5倍である。これを受けて,国連事務総長グテーレスは,「地球温暖化の時代は終わり,地球沸騰化の時代が始まった」と危惧している(Guterres,2023) 。
2. current status of major greenhouse gases on a global scale
地球規模で温室効果ガスの排出量やその原因の定量化を目指す地球炭素プロジェクト(GCP)という組織がある。2001年に設立された国際研究計画である。そのGCPが主要な温室効果ガスのCO2,CH4,N2Oの現状を以下のように公表している。
1)CO2(Friedlingsteinら,2023)
人間活動によるCO2排出の主な排出源は次の二つ,すなわち,①化石燃料の燃焼(詳細は表1の注2参照。以下,EFOSと略)と,②土地利用の変化と林業分野からの排出(詳細は表1の注3参照。以下,ELUCと略)である。2013年から2022年の10年間の平均年間CO2排出量は,炭素(C)換算で,EFOSとELUCの合計10.9Gt(ギガトン=10億トン)だった(表1) 。
一方,CO2の主な吸収源も二つで,①陸域による吸収(詳細は表1の注4参照。以下,SLANDと略)と,②海洋による吸収(詳細は表1の注5参照。以下,SOCEANと略)である。両者の合計6.1Gtが平均年間CO2吸収量(C換算量)だった(表1) 。
大気中のCO2量は,上記の排出量と吸収量の差で与えられ,排出量のほうが吸収量より4.8Gt上回っていた(表1) 。これが大気中のCO2増加を裏付けている。とくに注意すべきことは,現在の大気中CO2の増加が,過去200万年間で前例がないことであり,その増加速度が過去80万年間のどの時期よりも,少なくとも10倍も速いことである。まさに,地球沸騰化時代が始まっている。
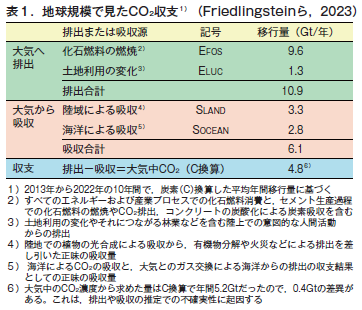
2)CH4(Saunoisら,2020)
CH4は地球を温暖化させる能力(地球温暖化指数)がCO2の34倍とされ(Myhreら,2013) ,地球温暖化に大きく影響する。地球規模での平均年間CH4総排出量は,2008年から2017年までの調査では737Tg(テラグラム=100万トン) ,人為起源と自然起源,それぞれが50%ずつだった(図1) 。これより前の10年と比べ,自然起源の排出量は大きな変化がなかったのに対して,人為起源の排出量は10%の増加で,増加傾向が継続している。
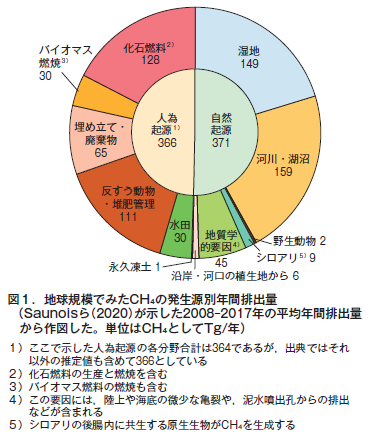
人為起源のうち,農業にかかわる要因では反すう動物と堆肥管理からの排出量が最も多く,人為起源排出量の30%を占めた。反すう動物は,採食した飼料を第1胃で嫌気的に消化する過程で,メタン生成菌が生成したCH4を,あい気(げっぷ)で体外に排出するからである。水田からのCH4排出量も多い。水田や湿地は酸素が不足した(嫌気的)条件にあり,そこで土の有機物分解がすすむと,最終産物としてCH4が大気へ排出される。
水田を冬期も湛水状態で維持し,湿地に依存する多様な生物の生息地として利用すること(「ふゆみずたんぼ」といわれている)が,生物多様性保全のために推奨されることがある。しかし,長期にわたる湛水状態で,一般の水田よりもCH4排出量が多くなる(吉田ら,2010) 。それゆえ,温室効果ガスの排出という面から見ると,「ふゆみずたんぼ」は必ずしも環境保全的であるとはいえない。
3)N2O(Tianら,2020)
N2Oは地球温暖化指数がCO2の298倍あるだけでなく,オゾン層の破壊にも関与するガスである(Myhreら,2013) 。2007年から2016年の10年間で見ると,人為起源の排出量は,自然起源も含めた地球規模の平均年間総排出量17.0Tgの43%だった(図2) 。1980年代と比較すると,自然起源の排出量は3%減少したのに対し,人為起源の排出は30%も増加していた。人為起源の排出量のうち52%が農業からの排出だった。その平均年間排出量3.8Tgには,土から2.3Tg,草地に表面散布された堆肥から1.2Tg,堆肥の管理作業から0.3Tg,水産養殖から0.1Tgの排出量が含まれている。
この農業分野のN2O排出は,作物栽培のために化学肥料や堆肥などの有機質肥料で与えられた窒素に由来する。農業からのN2O排出抑制は,地球規模から見て重要である。しかし前回も指摘したが,その抑制対策を見つけるのは容易ではない。
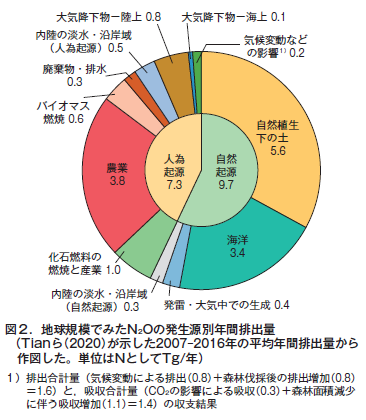
−第6章− 劣化する世界の土
第31回 危機に瀕する世界の土−その1
古代文明の崩壊と土の劣化
令和6 (2024) 年 5月号 (第760号)
From the 25th to the last issue of this series, it has been stated that agriculture can have a negative impact on the environment, depending on how it is conducted. Therefore, it is important to conduct agriculture in an environmentally friendly manner. This is because agriculture cannot be stopped because it produces food for people.
農業の作物生産の場を支えているのは土である。しかし,その土が荒廃して作物生産に適さなくなってしまう現象(これを土の劣化という)が地球規模で広まり,すでに土の3分の1が劣化しているという(Silva,2014) 。まさに土が危機に瀕している。それはなぜなのか,土の劣化の現状や原因を今回からしばらく探ってみたい。
1. indifference to the soil will destroy civilization
About 10,000 years ago, primitive man acquired the wisdom to secure food through agriculture. Once the foundation for stable food production was established through agriculture, the ability to support the population increased. Ironically, however, it also made it impossible to return to a hunter-gatherer lifestyle. The food supply of the increased population could no longer be supported by hunting and gathering. The agricultural and sedentary lifestyle gradually flourished as people left the natural ecosystem, created artificial ecosystems, and built settlements. This is where the seeds of ancient civilization were planted.
しかしこの文明の繁栄は,30から70世代(800から2000年)以上長続きすることはなかった。その根本原因は,自然生態系を無視し,土の肥沃度維持に無関心だったため,食料を持続的に生産できなくなったことにあると,カーターとデール(1975)はその名著「土と文明」で指摘している。彼らの指摘の一部を以下で見てみよう。
1) The Fertile Crescent, an example from Mesopotamia
「肥沃な三日月地帯」とは,チグリス川とユーフラテス川の流域に,その上流地帯からヨルダン川流域,死海までの低地帯のことをいう。かつてこの地域は「乳と蜜の流れる土地」とさえいわれた肥沃な土があった。この地域は降水量が少ない。そのため,作物栽培にはチグリス,ユーフラテス両河川から水を引き込むかんがい(灌漑)が必須だった。両河川上流にあるアルメニア高原は豊かな森林だった。しかし文明に触発されて人が集まると,燃料や建材用に森林が伐採され,家畜が過放牧された。森林を失った高原の土は水を保持しきれず,表土が侵食されて土砂が河川に流れ込み,かんがい用水路に堆積した。用水路の機能を維持するのは,奴隷労働による土砂の除去だった。
However, the peoples attacking Mesopotamia were indifferent to the irrigation canals. Eventually, the irrigation canals were blocked by earth and sand, rendering them unusable. The decisive blow came when the nomadic Mongols attacked the region. The nomads did not understand the importance of irrigation and completely destroyed the canals. Even worse, in this region of high evaporation, when irrigation connected the groundwater to the surface, a tremendous amount of salt was introduced and accumulated on the surface, causing the soil to become salinated. This salinization completely destroyed the soil's ability to produce crops.
食料生産に不可欠なかんがい水確保の水路が土砂で埋められたこと,土が塩類化しやすかったこと,これらの要因がメソポタミアでの食料の持続的安定供給を困難にして,人口扶養能力を低下させた。こうして文明が衰微していった。

(2) Example of the Nile River Basin, Egypt
自然を生かした土の肥沃度の維持
同じ古代文明発祥の地でもエジプトのナイル川流域は,メソポタミアとは事情がちがう。ナイル川は毎年正確な周期で増水と減水をくり返す。おもな水源が,エチオピア高原と中央アフリカの高地の雪解け水だったからである。年に一度,夏に必ずやってくる洪水は,古代エジプトに実りをもたらした。
First, an enclosure is built along the Nile River basin, similar to the footpath between rice paddies. In this enclosure, muddy water that overflows during the flooding season is stored like rice paddies before planting (called flooded irrigation). After a few weeks of irrigation, the fertile silt (fine sand) contained in the muddy water settles on the ground, and the water permeates the soil sufficiently. The excess water is then drained away, and wheat and other seeds are sown in the fertile muddy soil. People of that time, who thought in harmony with nature, did not try to conquer the enormous water energy of the Nile during the flooding season. Rather, they established a waterlogging irrigation technique that made good use of nature. However, this technology also had its drawbacks. It was a weakness in that it could only be planted once a year during the period of the Nile's rising waters.
通年かんがいへの転機
19世紀以降になると,エジプトでは綿花の輸出が計画された。しかし,綿花はナイル川の減水期(3〜4月)に播種され,10月に収穫される夏作物である。このため,増水期の水を利用できない。そこで,減水期の低い水位の水を利用するため,深いかんがい用運河が掘られた。こうして,ナイルの水量の増減に関係なく,水を通年利用する環境が整った。
この通年かんがいへの熱い思いが,後の1903年,イギリスの援助によるアスワンダムを完成させた。このダムの利用で安定した農業生産が約束された。そのおかげで人口が大きく増え,1882年に700万人だった人口が,70年後の1952年には2,000万人に達した。ところが,人の生活のための土地利用が進むと,原流域の山林が伐採され,家畜の過放牧もはじまった。こうした土地利用の変化で,ダムに運ばれる土砂が多くなり,ダムは土砂で徐々に埋められ,貯水機能が低下した。
ダムの機能低下はかんがい不能をもたらし,作物生産の減少につながった。そこでアスワン・ハイダムの建設が,ソビエト(現在のロシア)の援助で1960年に着工,10年後に完成した。これによって通年かんがいの農地ができ,作付けも年に数回可能となり,作付面積が大幅に拡大した。
ダムの功罪と自然の摂理
アスワン・ハイダムの完成は水問題を解決したかにみえた。しかし,張り巡らされたかんがい用水路に水生カタツムリが大発生,それを中間宿主とする寄生虫ビルハルツ住血吸虫の蔓延と,土の塩類化が始まった。
The annual Nile floods wash away the salts that tend to accumulate on the earth's surface. Moreover, they carry nutrient-rich fertile silt from upstream and deposit it on top of the old soil. This naturally overcame the salinization of the soil and maintained its fertility. This was the ancient natural order of things that supported the "fertile lowland soil of the Nile.
In modern times, however, people have attempted to control the flow of the Nile themselves. The Aswan High Dam, which was supposed to have been built to stabilize agriculture, caused salt damage and parasites. This is a tragedy of irrigated agriculture that failed to take advantage of the natural order of things.

2. how to apply lessons learned
古代文明の衰退が教えることは,食料生産の基盤である土を保全しないで食料の確保はあり得ず,高度な文明も維持できないという事実である。太平洋の孤島イースター島での資源の枯渇による文明の崩壊もまた,現代の私たちに貴重な教訓を提供している(ポンティング,1994) 。これらの教訓をどう生かすか,現代の私たちに問われている。
−第6章− 劣化する世界の土
第32回 危機に瀕する世界の土−その2
不適切な人間活動が土を劣化させる
令和6 (2024) 年 6月号 (第761号)
Soil is the foundation of food production. Without soil conservation, food security is impossible and advanced civilization cannot be maintained. In my previous article, I said that we must ask ourselves how we can make the most of this lesson today. Unfortunately, I do not believe that this lesson is being fully applied. This is because soil degradation is still progressing around the world. In this issue, I would like to discuss inappropriate human activities that are the main cause of this degradation.
1. soil degradation due to human activities
Degradation of food-producing farmland refers to the phenomenon in which the soil is degraded and crop productivity is significantly reduced or eliminated as a result of inappropriate soil management and excessive deprivation of the soil in order to increase productivity.
土は環境の産物である。土は与えられた環境の下で最も安定する方向に変化し,つくりあげられる。それゆえ人間活動がその変化の範囲内であるかぎり,土自身が劣化していくことはない。人間活動が環境による変化以上の変化を土に与えたとき,土が劣化する。人間活動による土の劣化は,やや古いデータではあるが,世界で20億ha程度,全植生地のおよそ17%に達するという(図1) 。
最近,これと同様のデータの公開がない。しかし状況はさらに悪化しているようだ。国連食糧農業機関(FAO)事務総長シルヴァが国際土壌デーと国際土壌年の発足にあたり,世界の土の3分の1が劣化しているとの驚くべきメッセージを発表している(Silva, 2014) 。
Major human activities that degrade the soil include improper agricultural management through over-cultivation, overgrazing that allows livestock to graze more than the regenerative capacity of wild grasses, and over-cutting of forests.
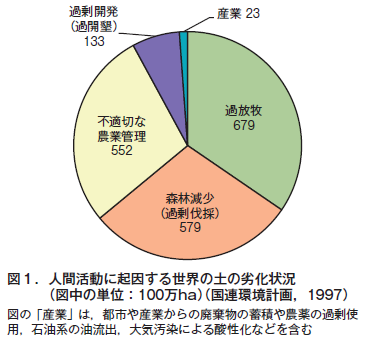
2. human activities that degrade agricultural soil
1)不適切な農業管理過剰耕作
Convenient land suitable for agriculture is cultivated and turned into farmland at an early stage. However, the more such land is cultivated, the more people live there. In order for humans to live, land is necessary. Industrial land is also necessary. At present, most of the land suitable for agriculture has already been developed, and the development of agricultural land has reached its limits. Therefore, as the population continues to grow, the world's per capita cereal production area continues to decline. As a result, increased food production has to be expected on a per-unit-area basis.
途上国のように粗放な農業地域では,養分補給や土の管理が不十分のまま過剰耕作がくり返され,土の酷使がすすむ。焼畑移動耕作も,かつては土の肥沃度と森林再生のいずれもが十分に回復してから再利用した。しかし,最近はそれができなくなってきた。人口が増加したため,移動耕作のための土地面積が減少したからである。とくに乾燥地域や半乾燥地域での過剰耕作は土地の砂漠化につながる(伊ヶ崎,2015) 。さらに,この地帯での不適切なかんがいの導入は,メソポタミアで見た土の塩類化を招きやすい。
On the other hand, intensive farming areas tend to provide more nutrients than necessary in the hope of increasing yields. This leads not only to environmental pollution but also to soil salinization caused by excess nutrients, making it impossible to grow crops. This improper management of agriculture leads to soil degradation.
(2) Overgrazing
Developing countries in Asia and Africa have used the wild grasses of the land for grazing use by livestock. This is because they provide a living food reserve for livestock. Their manure is used as a source of nutrients for the soil, and dried manure is sometimes used as fuel. However, as the number of grazing livestock increases with population growth, grazing livestock forage for more wild grasses than they regenerate, resulting in a decrease in the density of wild grasses, which exposes the soil. This is the state of overgrazing.
Under overgrazing conditions, not only is the soil exposed, but it also becomes hard and compacted by the treading pressure of livestock. The hard soil surface prevents rainwater from infiltrating the soil, and surface runoff erodes the topsoil, accelerating degradation. Wild grasses also play an important role in helping the soil retain water. However, when wild grasses are lost from the soil due to overgrazing, the soil becomes arid and desertified.
(3) Loss of forests
Forests also play an important role in retaining soil moisture. However, population growth in developing countries increases the demand for wood for housing and fuelwood, and the use of wood expands to include important forests. The expansion and inappropriate use of slash-and-burn cultivation areas and conversion of land to pastureland also lead to the gradual decline of forests. The loss of forests that follows the decline also contributes to soil degradation. In particular, the loss of forests in tropical rainforest areas causes further soil degradation due to soil erosion.
2020年の地球上は,陸地のおよそ31%に相当する41億haが森林(植林地を含む,以下同じ)で覆われている(FAO,2021) 。1990年から2020年の30年間で比べると,増加した森林面積は6,860万haだったのに対し,2億4,610万haが消失している。つまり,この30年間で世界から失われた正味の森林面積は1億8千万haに達し,わが国の国土面積(3,778万ha)の約5倍に相当する。これは,毎年600万haもの森林が消失していったことになる。ただし,世界の森林消失面積は全体でみると,1990年以降,減少しつつある。
森林消失面積が大きかったのは南アメリカ地域で,1990年からの30年間に1億2,950万haも消失した。このうちの大部分はブラジルで失われた9,230万haである。ブラジルでは平均するとこの30年間,毎年308万haも消失したことになる。アマゾンの熱帯雨林地帯での違法で不適切な過剰伐採が主な要因である。この消失速度は,わが国の全森林面積(2,494万ha,2020年FAO)が,およそ8年で失われることを意味する大きな値である。アフリカ地域でも1990年以降の30年間で,1億1千万haの森林が消失した。他の地域と異なり,1990年以降の10年ごとの消失面積に減少傾向がなく,直近の2010年からの10年間には3,940万haが失われている。
一方アジアは,1990年からの30年間に森林面積が5,270万ha増加している。これには,中国が植林活動によって同じ30年間に森林面積を6,280万ha増加させたこと(図2) ,またインドも同じ期間に822万haも拡大させた影響が大きい。逆にインドネシアでは,同時期に2,640万haも森林面積を失っている。インドネシアの熱帯雨林や泥炭林で,日本などへの製紙原料として過剰伐採が続けられた結果である(相楽,2021) 。
Excessive logging in tropical rainforests in Brazil, Indonesia, and other countries has a significant negative impact not only on the soil but also on the global environment. I will discuss this on another occasion.

−第6章− 劣化する世界の土
第33回 危機に瀕する世界の土−その3
塩類集積による土の劣化とそのリスク
令和6 (2024) 年 7月号 (第762号)
In the previous issue, we discussed that soil degradation is progressing around the world and that the main causes are inappropriate human activities, especially over-cultivation, over-grazing, and over-logging. The main cause of soil degradation is the accumulation of salts (salinization) in the soil caused by inappropriate agricultural management.
1. soil degradation and salt accumulation
According to the Food and Agriculture Organization of the United Nations (FAO, 2021), in 2015, the world's agricultural land area (the sum of crop cultivated land (= all cultivated land) and permanently used grassland) was 4.76 billion ha, of which 34% was soil degraded due to human factors. Furthermore, the area of farmland has decreased by 110 million ha in the 15 years since 2000. On the other hand, the total cultivated land where crops are cultivated was 1.56 billion ha in 2015, an increase of 0.63 billion ha since 2000. Most of this increase, 0.49 billion ha, was in the area of land with irrigation facilities. As will be discussed below, this is an indication of the high productivity expectations of irrigated agriculture.
しかし全耕作地でもその31%に当たる4.7億haは,人為的要因によって土が劣化している(表1) 。とくに灌漑耕作地ではその割合が48%,1.5億haになっている。灌漑耕作地で土を劣化させる最大の要因は塩類集積である。世界で塩類化によって耕作放棄される灌漑耕作地は,毎年,150万ha(岩手県面積とほぼ同じ)と推定されている。
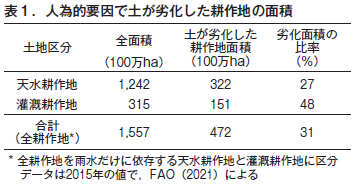
2.塩類集積の二つの型
There are two types of salt accumulation in soil. One is salt accumulation that occurs naturally over a long period of time when the parent rock, which is the raw material of the soil, contains high concentrations of soluble salts, or when groundwater with high salt concentrations is present near the surface in arid regions. This is called primary salt accumulation. In this case, salts have naturally accumulated in the soil for a long time, and there is not much use of the land for agriculture. The other case we are considering here is salt accumulation caused by human factors such as inappropriate farmland management in irrigated agriculture, which is called secondary salt accumulation.
FAO(2015)によれば,世界の陸地面積は127.8億haあり,その内の6.5%(8.3億ha)で土が塩類化しているという。ただし,このうちのおよそ90%以上は,一次的塩類集積による土の劣化と推定されている(図1) 。農業の土地利用から見て問題となるのは,二次的塩類集積によって発生する土の劣化である。

3. highly productive irrigated cropland
乾燥地域や半乾燥地域はそもそも雨が少ない。前者の年間雨量は200mm以下,後者でも夏雨地帯では年間雨量800mm以下,冬雨地帯で500mm以下である。いいかえると,この地域は晴天の機会が多い。これに水や養分があれば,作物の光合成が十分におこなわれるため,この地域の土地の作物生産性が高くなるのは当然である。事実,灌漑耕作地の穀物収量は雨水だけに依存する耕作地(天水耕作地)のおよそ2倍にもなっている(FAO,2011) 。したがって,灌漑耕作地は世界の全耕作地面積のおよそ20%(2015年,3.2億ha,表1)にすぎないにもかかわらず,世界の穀物生産量の40%が生産されている(FAO,2021) 。
4. soil salinization accelerated by coarse irrigated agriculture
However, once irrigated agriculture is started in arid and semi-arid regions, sustainable agriculture cannot be established without securing irrigation water in the future. This is because rainfall is scarce in these regions.
Even worse, these areas often have clay layers and other poorly permeable soil layers (impermeable layers) in the relatively shallow parts of the soil layers. When large amounts of irrigation water are poured into the soil with inadequate drainage channels, excess water stagnates in and around the farmland, causing water logging (waterlogging and overwatering) where the land is covered with water like a paddy field, or a temporary groundwater table is created in the soil. When soil is placed under such conditions, fine gaps (capillary pore spaces) in the soil connect the surface soil to the groundwater. When the excess water recedes and the topsoil appears, the water in the soil moves to the ground surface in this region where evaporation is very active. When water evaporates at the surface, dissolved nutrients and other salts remain in the surface layer of the soil and accumulate on the soil surface. Because there is little rainfall, the accumulated salts are washed away again and do not percolate into the soil. This accelerates the accumulation of salts in the soil.
Therefore, the introduction of rough irrigated agriculture in these arid and semi-arid regions without proper drainage channels leads to the tragedy of salinization of the soil. As already mentioned in this series of articles, this should have been learned by mankind from the history of the decline of ancient civilizations. However, that lesson has not been applied.
Concerns associated with competition for water resources
水(淡水)資源は,農業だけでなく工業や地域住民など多くの関係者で利用されている。これらの全ての関係者によって取水された淡水総量の再生可能な淡水資源総量に対する比率を水ストレスという。北アフリカはこの比率が100%を超えて重大な水不足にあり,中央アジア,中東から中央アジアの各地域は水分ストレスが70〜80%と大きい(FAO,AQUASTAT,2021) 。これらの地域では,農業部門による取水量が総取水量の75%以上にもなっており,灌漑農業自身が地域の水分不足を高める要因になっている(図2) 。
さらに,灌漑耕作地のおよそ40%は地下水に依存している(FAO,2011) 。とくに水の補給がない地下帯水層での水分枯渇は深刻な懸念である。将来の水不足の時代に,農業と他部門との水資源の競合がさらに激しくなることも想定される。このため,灌漑耕作地への水供給の持続性には不安が大きい。灌漑農業の高い生産性は,世界の食料安全保障に大きな役割を果たしている。それだけに,灌漑農業の高い作物生産性を持続的に維持することの難しさは,未来の食料生産への不安でもある。
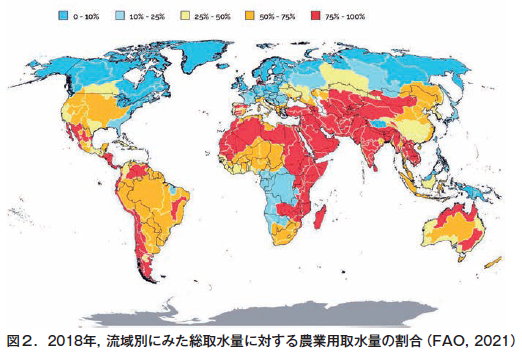
−第6章− 劣化する世界の土
第34回 危機に瀕する世界の土−その4
侵食による土の劣化
令和6 (2024) 年 8/9月合併号 (第763号)
Following the previous three articles, the soil degradation discussed in this article is the damage caused by erosion. There are two types of erosion. One is water erosion, in which the topsoil is eroded from the surface by water, and the other is wind erosion, in which the topsoil is eroded by wind. This erosion is the most serious damage to soil in the world.
1. History of Soil Deterioration
The concept of "sustainable development," or development that satisfies the needs of future generations while also satisfying the needs of present generations, first appeared in 1987. This concept was presented in the final report "Our Common Future" issued by the World Commission on Environment and Development (abbreviated as the Brundtland Commission after its chairman), which was established by the UN General Assembly in 1984. The Commission's problem was that "economic growth and development are necessary to solve problems such as the world's population explosion and poverty. However, if this leads to environmental destruction and resource depletion, sustainability will be lost. Therefore, the conclusion was that "sustainable development" was the key to solving these problems.
この環境破壊や資源の枯渇で最も注目されたのが土という資源だった。当時,すでに土の劣化が問題視され,とくに土の侵食が人類の将来の繁栄を損なうとの主張が出始めていたからである。しかし,そうした主張には土の劣化問題が深刻な場所と,そうではない場所がどこにどれくらいあるのか,土を劣化させた原因が何かということへの具体的な裏付けがなかった。その裏付けとなる情報収集のために,ブルントラント委員会の最終報告書が出されたとほぼ同時に,国連環境計画(UNEP)が国際土壌情報センター(ISRIC)と「土の劣化の地球規模評価(GLASOD)」というプロジェクト(3年間,1987〜1990)を立ち上げた。
2. type and area of soil deterioration
このGLASODには世界中の多くの土壌学者が参加し,世界の陸地面積およそ130億haを対象にして,劣化の原因,種類,範囲,程度などが調査された。その結果は1991年に,劣化の種類別に縮尺1000万分の1の世界地図として公開された。その面積に関する結果が表1である(Oldemanら,1991) 。
Table 1 shows that 15% of the world's land area, or 1.96 billion hectares, was degraded by anthropogenic factors. The anthropogenic factors that contribute to soil degradation have already been described in the June issue of this series (Vol. 32). The main factors are inappropriate over-cultivation, overgrazing, and excessive deforestation.
GLASOD classifies degradation into four categories according to its status: water erosion, wind erosion, chemical change, and physical change. Of this total, 84%, or 1.64 billion ha, was soil erosion damage. Water erosion accounted for 1.094 billion hectares, and wind erosion for 548 million hectares. It is clear that the cause of soil degradation in the world at that time was erosion triggered by anthropogenic factors.
なお,この当時の土の劣化のうち,塩類化は0.76億haに過ぎなかった(表1) 。しかし,前回述べたように, 2015年の世界では8.3億haが塩類化していた(FAO,2015) 。20世紀末から現在まで,塩類化の進行のすさまじさがわかる。
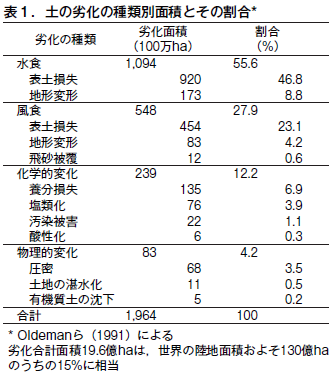
3. natural and accelerated erosion
Soil erosion damage caused by human factors is often triggered by improper soil management. Even in the absence of such conditions, topsoil gradually moves downward on sloping land. This is called natural erosion and is a natural process that produces fertile lowland soil.
しかし,地面を覆う植物を人為的に除去して耕地化すると,土の侵食速度は自然侵食の数百倍もの早さで激しくなる。この人間活動の影響を受けた侵食を加速侵食という。自然侵食と比較してその被害は大きい。アメリカ土壌保全局ができた1935年からまもなくの研究は(Bennett, 1939) ,表土の露出度の大きい綿花の栽培は侵食被害が大きく,抑制には草地としての土地利用が最も優れていることを明らかにしている(表2) 。牧草が土を覆い,加速侵食を阻止するからである。
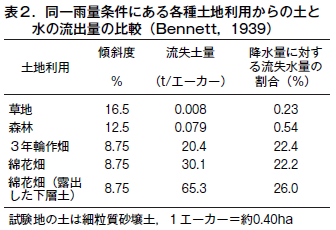
4. erosion and salinization damage observed in Central Asia
The history of soil degradation caused by erosion is long. In particular, the U.S. experienced large-scale erosion damage three times from the period of western settlement to the 1930s, and the Soil Conservation Service was established in the Department of Agriculture based on lessons learned from this experience. However, soil erosion in the U.S. continues to this day.
アメリカと同様に,大規模風食被害を経験したのが,中央アジアのカザフスタンとタジキスタンである。旧ソビエト連邦の全耕地面積の20%に相当する4,000万haの土が被害を受け劣化した。カザフスタンとウズベキスタンの国境地帯は,旧ソビエト連邦の「自然改造計画」によって,草原から綿花畑に改造された。乾燥地域につくられた綿花畑には灌漑水が必要で,パミール高原と天山山脈の融雪水を水源とするアムダリア川とシルダリア川から取水された。その結果,ウズベキスタンの綿花生産は世界5位になっている。
しかし,その結果として両河川の水が注ぎ込んでいたアラル海(広さは琵琶湖のおよそ100倍)が干上がった。両河川からの取水によってアラル海への水供給が途絶えたからである。アラル海はもとの面積の10%にまで縮小し,アラルカン砂漠になってしまった。この砂漠から風食で有害化学物質を含む砂塵と塩分が,年間7,500万トンも飛び散っている(星野,2011) 。アムダリア川から取水された水利用も不完全で,土が塩類化し,耕作が放棄されるまで劣化してしまった。まさに,20世紀最大の環境破壊である。

−第6章− 劣化する世界の土
第35回 危機に瀕する世界の土−その5
酸性雨による土の劣化
令和6 (2024) 年 10月号 (第764号)
In the latter half of the 20th century, air pollution became a serious pollutant as human economic activity increased. Acid rain generated by air pollution is another cause of soil degradation. Even today, when air pollution control measures have become widespread, there are still concerns about the damage caused by acid rain, especially in forested areas.
1.酸性雨と酸性降下物−用語の整理
大気が清浄で汚染ガスが含まれていなければ,雨は空気中の二酸化炭素(CO2)を溶かしながら降ってくる。このため,雨水のpHは飽和炭酸水のpHである5.6程度になる。ところが現実には,大気にさまざまな汚染物質が含まれているため,pHは5.6より低いことが多い。このpH5.6より低い降雨を酸性雨という。
しかし,一般に使用されている酸性雨という用語には雨だけでなく,霧,雪などの形態で降下するもの(これらを総称して湿性沈着または湿性降下物という)の他に,晴れた日でも風にのって沈着する粒子状(エアロゾル)やガス状の酸(これらを総称して乾性沈着または乾性降下物という)も含めることがある。湿性沈着と乾性沈着の両方を含めて酸性降下物という(図1) 。
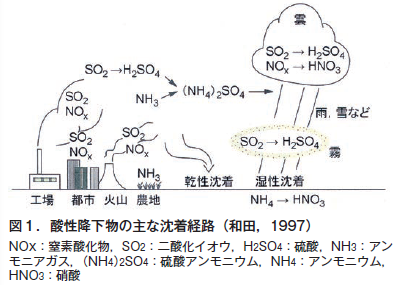
2. history of acid rain and air pollutants
酸性雨の歴史は,産業革命以降の人類による大気汚染の歴史と重なる。酸性雨という言葉は1872年に,ロバート・アンガス・スミスが,彼の著書『大気と雨−化学的気候学の始まり』で用いたのが最初である。彼はこの著書で,産業革命で大工業地帯を形成していたイギリス・マンチェスターとその周辺の石炭燃焼が大気を汚染し,それが酸性雨を発生させていると指摘した。その後もイギリスは大気汚染によって長期にわたり悩まされた。とくに19世紀のロンドンは被害がひどく,大気汚染によって死者さえだしている。
産業革命以降,産業活動が活発化し,石炭や石油など化石燃料の消費量が増加した。その結果,大気にイオウ酸化物(SOx)や窒素酸化物(NOx)が大量に放出されるようになった。これらの酸化
物が大気中で複雑な化学反応を経て,最終的に硫酸(H2SO4)や硝酸(HNO3)などを生成し,よりpHの低い強酸性の雨となって地上に戻ってきたもの,それが酸性雨,酸性降下物である(図1) 。
この他,酸性雨関連大気汚染物質には,図1の農地から発生するアンモニアガス(NH3,主に家 畜ふん尿からの揮散に由来する)や,図1には示されていない海塩性の塩素(Cl,荒天で海水中に巻き込まれた空気の気泡が海面で破裂して粗大粒子が生じ,それが大気に巻き上げられて発生)なども含まれる。塩素は大気中で塩酸となって降下する。
3. Acid Rain in Japan
わが国では環境庁(現,環境省)が1983年以降全国各地で酸性降下物の観測を続けている。最新の2022年度の酸性雨調査結果では,湿性沈着を調査した全国19地点の調査5年間(2018〜2022年度)の平均降水pHは4.95と強酸性だった。最低は鹿児島県屋久島で観測されたpH4.69,最高は長野県八方尾根のpH5.21だった(環境省,2023) 。
4. environmental damage caused by acidic fallout
酸性降下物による被害は具体的に眼にすることができる(図2) 。

しかし,それ以上に危惧されることは,眼にみえず静かに被害が拡大していくことである。土の酸性化はその1つである。強酸性の酸性雨は,土に保持されているカルシウムやマグネシウムなどの陽イオン類を洗い流し,土の酸性化を促進する。これが土の劣化や生物の多様性を失わせる。こうした酸性降下物による被害を受けやすい地域として指摘されたのは,ヨーロッパ,アメリカ東部沿岸地帯,そしてインド西岸地帯や中国南東部などだった(図3) 。
森林や河川,湖沼への酸性降下物による被害では,スウェーデン,ノルウェー,カナダの河川,湖沼での「アシッド・ショック」とよばれる被害が大きい(石,1992) 。湿性降下物として降り積もった雪は雪解けとともに一斉に溶けだし,強酸性水となって河川や湖沼に一気に流れ込む。すると,河川や湖沼のpHが急激に低下して強酸性を示し,水生動物に大きな被害をもたらす。これがアシッド・ショックである。
5.酸性降下物による森林被害
森林の被害は,酸性降下物による直接的な害作用だけでなく,酸性降下物に由来する養分の富化による被害もある。わが国の酸性降下物に由来する窒素は年間8kg/ha程度であった(村野,1993)。この量は農作物へ与えられる窒素量の5〜10%程度で無視できる量ではない。もともと,森林には作物の養分を与えることはない。このため,窒素などの養分を豊富に含む降下物が森林に降下すると,樹木の生育が一時的に旺盛になり,貧栄養状態の森林の土から,樹木が積極的に養分を吸収する。その結果,酸性降下物から供給されない養分は,土から枯渇し樹木の生育を阻害する。場合によっては,降下物に由来する窒素の供給によって葉や枝が茂りすぎて,樹木全体としての窒素栄養の調和が失われる。このように,降下物による養分の富化が自然の物質循環に基づいた樹木の生育を撹乱し,それによって森林被害が発生する。
Other acidic substances are deposited on leaves in an acid fog-like state, and when the water gradually evaporates and becomes concentrated, the leaf surface is damaged. When acidic substances are deposited on the leaves again, the damage is expanded, and so on, in a chain reaction. As this progresses, the trees die. When forest soil is exposed due to such forest damage, it accelerates soil erosion damage and leads to soil degradation.
わが国でも,関東地方でのスギ枯れや,丹沢山系の大山でのモミが枯れるといった被害は,ヨーロッパの針葉樹林の酸性降下物による被害と類似した被害で注目された。しかし,その後の調査で酸性雨被害というよりも,光化学オキシダント等のガス状大気汚染物質による被害ではないかと考えられている(古川,1989) 。森林被害のすべてが酸性降下物によると決めつけることはできない。
−第6章− 劣化する世界の土
第36回 先進国経済が途上国の土や資源を収奪する
−その現実と環境破壊の事例から学ぶこと−
令和6 (2024) 年 11月号 (第765号)
In the past five articles, I have described the current state of the world's soil degradation and the crisis it is endangering. The main cause of soil degradation has been inappropriate human activities on agricultural lands. However, there is a deeper root cause hidden in the soil degradation caused by inappropriate human activities. The hidden cause is the economic structure in which the globalization of developed countries' economies deprives emerging and developing countries of labor and resources. Saito (2020) called such affected areas and inhabitants the Global South.
In this issue, I will present examples of economic exploitation by developed countries that have caused soil degradation and environmental destruction in the Global South. In each case, the environmental destruction was caused by the expropriation of labor and resources in the name of producing raw materials necessary for our daily lives.
Example of oil palm plantation in Indonesia
アブラヤシはパームヤシとも呼ばれ,果実の果肉からパーム油が,種からパーム核油が生産される。両者は主成分が異なる別物である(図1) 。

Palm oil is not only inexpensive, but is also resistant to oxidation. For this reason, it is widely used in processed foods, snacks, fast foods, French fries, ice cream, chocolate, margarine, soap, detergents, cosmetics, and other products. However, in Japan, the labeling of food ingredients only mentions "vegetable fats and oils. Therefore, it is difficult to be aware of the fact that we depend heavily on palm oil in our daily lives.
The cultivation area of oil palm, the raw material of palm oil, has doubled in the 21st century. In Indonesia, the largest palm oil-producing country, the oil palm cultivation area was only 70,000 ha in 1961. By 2018, it was 6.78 million hectares, a rapid increase of about 100 times. The expansion of cultivation area was supported by the overexploitation of tropical rainforests. Overexploitation has led to rapid deforestation. Not only that, the rapid increase in palm oil production had a destructive impact on the livelihood of the local people, who had depended on the natural environment for their livelihood.
熱帯雨林を切り拓いた後のアブラヤシ農園の造成や農園管理,さらに収穫方法などいずれも,現地の小規模農家の伝統的技術が採用されなかった。国が主導したのは,アブラヤシ認証制度に基づく「生産技術の手引き」だった。それは世界の植物性油脂の需要増大に応え,パーム油産業の国際競争力を高めることを目的とする「手引き」だった。すなわち,農園ではアブラヤシ単一栽培とし,労働力と資材(化学肥料や農薬)を多投する集約的なプランテーション技術だった(寺内,2021) 。
しかしこの技術に対応できる小規模農家は少なく,実際は極めて不適切に集約栽培が実施されてしまった。このため農園の土は侵食被害を受け,乱用された化学肥料や農薬が河川に流出して川魚を減少させてしまった。この川魚は地域住民の貴重なタンパク質源だった。住民はそのタンパク質源を摂取できなくなり,別のタンパク質を入手するには費用が以前より多く必要となった。その費を作り出すために野生生物,とりわけオランウータンやトラなど絶滅危惧種の違法取引に手を染めるようになった。こうして住民の生活は大きく変化してしまった(斎藤,2020) 。
This tragedy is occurring in the shadow of our demand for cheap palm oil production.
2. deforestation for papermaking raw materials, examples in the manufacturing process of avocado, soybeans, cacao, etc.
パーム油の例を紹介したインドネシアでは,国内最大の製紙メーカーが日本など120カ国以上に紙を輸出するために,原料をスマトラ島やカリマンタン島などの熱帯雨林や泥炭林に求めた。この時,メーカーは現地住民との間で「自由意志による,事前の,十分な情報に基づく同意(FPIC=Free,Prior and Informed Consent)」を実施することになっていた。しかしそれは名ばかりで,FPICを実施したということで原料調達の森林伐採がおこなわれ,地元住民との間で紛争が発生している(相楽,2021) 。わが国でも,FPIC認証のコピー用紙が販売されている。いかにも環境に配慮した用紙であるかのようだ。しかしその背後に森林伐採のしわ寄せ被害をうけている現地住民がいる。
中南米でのアボガドのプランテーション開発では,生産拡大のための違法な森林伐採に加えて,アボガドがとくに必要とする水要求に応えるために水が過剰消費され,現地での水不足をもたらしている(六辻,2021) 。
私達の食事に重要な大豆の主要輸出国ブラジルでは,大統領が熱帯雨林の乱開発を黙認し,その開発によって原住民たちが住んでいた土地から追い出されている(Chandrasekhar,2020) 。私達が美味しいと味わうコーヒーやチョコレートの原料を生産するために,コーヒー農園やカカオ工場では,子供達(5〜17歳)が自身や家族の生活のために安い賃金で労働している(図2) 。こうした農林水産業で働く子供達は世界でおよそ1億1,210万人,全世界で働く子供達1億6千万人のおよそ70%に相当するという(吉岡,2022) 。

3. case study in metal mining
アフリカのガボンでは金鉱山開発の名目で森林破壊が進み,それが人畜共通感染症の病原体を運ぶとされるコウモリなどの動物のすみかを破壊し,エボラ出血熱などの感染症まん延の原因を作り出している(斉藤,2020) 。再生可能エネルギーとして注目される風力発電の原動機(タービン)や,化石燃料の消費抑制に期待されるハイブリッド車の電池などに利用されるレアアース(希少金属レアメタルの一種で希土類ともいう)も,その採掘にともなって環境破壊を進行させる。太陽光発電パネルにはレアメタルが利用され,同様の環境破壊が発生している。世界最大の生産国・中国では,レアアースの生産過程で排出される有毒ガスや,カドミウムなどの重金属類が不純物として排出され,環境破壊がひどい状況にある(Biggs,2011) 。クリーンエネルギーを生産するために環境を破壊しているという皮肉な現実である。
4. the "cheap is good enough" mentality puts the burden of cost reduction on the weak.
Yamashita (2001) points out that, in response to consumers' demand for inexpensive food, "Just as each person has his or her own value, there is and must be a decent price for things. The cost reductions that make food inexpensive are achieved by externalizing the environmental impact of the cost reductions onto people and the natural environment somewhere far away.
We need to change our mindset not to use the spirit of the SDGs as a "pretty word" or an "alibi" for environmental concerns. We are called upon to change our consciousness, not to use the spirit of the SDGs as a "pretty word" or an "alibi" for our environmental concerns.
−第7章− 有機農業と慣行農業
第37回 農地は作物を栽培する土地である
−農地で生物の多様性をどう考える−
令和6 (2024) 年 12月号 (第766号)
So far, I have described the reality that man's excessive economic activities have not only brought the world's soil to the verge of degradation, but have also led to environmental destruction. Starting this month, I would like to change the topic to organic agriculture and compare it with conventional agriculture that has been commonly practiced. This month's theme is how to understand farmland and how to consider biodiversity in that farmland.
1. farmland is not a pristine natural ecosystem
About 10,000 years ago, people began what we now call agriculture. This beginning of agriculture and food production on farmland was also the beginning of environmental destruction in the sense that people added their hands to the pristine natural ecosystems that had been given to them.
農業が始まってからの農地では,そこで栽培される作物の生産性を最大にするために,人が作物にとって最適な生育環境を整える管理作業を続けてきた。例えば,慣行農業での水田という農地では,イネの生育が優先されイネ以外の様々な動植物の侵入を防ぐように人が管理する。したがって,生物の多様性は低く抑えられている(図1) 。
この努力は,一方で生物の多様性を守るという立場からみるとむしろ逆行している。農業が世界の生物多様性に対する最も深刻な脅威の1つとみなされているのは(Newboldら,2015) ,このためである。これに対し,有機農業は慣行農業と異なり,農地という人為的な生態系であっても生物の多様性を守ることに意義を見いだす。

2. organic farming aims to improve the quality of life and livelihood
わが国で有機農業といえば,単に化学肥料や農薬を使用せずに農産物を生産する農業とか,有機JAS規格を満たす農業と思われがちである。しかし,有機農業とはそのような外形で表現できるものではない。奥深い目標が有機農業にはある。世界の有機農業活動を国際的に束ねる組織である国際有機農業運動連盟(1972年フランスで発足,略称IFOAM:アイフォーム)は,2008年の総会で有機農業の定義と有機農業が目指すことを,以下のように宣言している。「有機農業は,土壌・自然生態系・人々の健康を持続させる農業生産システムである。それは,地域の自然生態系の営み,生物多様性と循環に根差すものであり,これに悪影響を及ぼす投入物の使用を避けて行われる。有機農業は,伝統と革新と科学を結び付け,自然環境と共生してその恵みを分かち合い,そして,関係するすべての生物と人間の間に公正な関係を築くと共に生命(いのち)・生活(くらし)の質を高める。」 (日本語訳はIFOAMによる)
Thus, organic farming is not only about producing healthy food, but also about the local environment, the living creatures including people who live there, and the way people should conduct agriculture. This is why organic farming is also strongly concerned with local environmental preservation and animal welfare (animal welfare). It never thinks that it is enough if it is good enough only for oneself.
3. it is difficult to balance crop production with protecting biodiversity on farmland
問題は農地で生物多様性を豊かに維持することと,その農地で目的の作物生産を高めることの両立が難しいことである。
わが国で水田を対象とした大規模実態調査の結果によると(Katayamaら,2019;片山ら,2020) ,生物の多様性は有機農業の水田のほうが慣行農業より優れていた(図2) 。ところが同じ調査で,イネの収量を両者で比較すると,有機農業の水田のほうが慣行農業よりも30%減収だった(図3) 。
The higher yields in rice paddies in conventional agriculture than in organic agriculture are the result of the farmland's objective to protect the growth of rice plants in preference to other plants and animals, and to increase yields. Organic paddy farming recognizes the significance of protecting biodiversity by allowing the plants and animals that congregate in the paddy fields to coexist, even at the expense of rice yield.
同様の比較を様々な作物について世界的な報告から検討した研究でも,有機農業の作物収量は慣行農業で栽培された同じ作物の75〜80%程度であった(De Pontiら,2012;Seufertら,2012) 。
ただし,このような農地での生物多様性と作物生産性との関係が両立しにくいのは,有機農業に限定された結果ではない。例えばイギリスの牧草地で,化学肥料として与える養分の種類や量(施肥量) ,さらに酸性改良の有無などの処理を160年以上も継続した試験の報告でも,同様に興味深い結果が指摘されている(Crawleyら,2005) 。
When nitrogen application was increased using a chemical fertilizer (sodium nitrate), which is less likely to cause acidification of pasture soil over time in this study, a species of grass that responded well to fertilizer nutrients became dominant among the many grass species, reducing diversity. However, hay yield increased. Conversely, when nitrogen application was reduced, there were no dominant grass species in the pasture, and many grass species grew and diversity increased. However, hay yield decreased.
In the end, it can be understood that biodiversity on farmland is not a characteristic of organic agriculture alone, but reflects differences in thinking about whether priority should be given to the growth of cultivated crops on farmland or whether coexistence with other plants and animals should also be tolerated.
4. the meaning of the coexistence of organic and conventional agriculture
With the world population projected to reach 9.7 billion by 2050, food production through agriculture will become increasingly important to protect human life. Conventional agriculture attempts to increase production (yield) per unit land area by creating an environment that is beneficial to the growth of crops grown on that land. These efforts are essential to ensure a stable supply of food for people from the earth's limited land resources.
On the other hand, organic agriculture is necessary for those who want to support and share the goals and ethics of organic agriculture, and for those who cannot eat produce produced by conventional agriculture with peace of mind due to concerns about chemical substances. However, it is necessary to recognize once again that it is difficult to balance crop production with the preservation of biodiversity, which organic agriculture values, in the ecosystem of farmland.
−第7章− 有機農業と慣行農業
第38回 有機農業の養分源・堆肥生産の課題
−堆肥生産には労力と土地が不可欠−
令和7 (2025) 年 1月号 (第767号)
農地で作物を栽培すると農地の土にあった養分は作物に吸収され,その作物が収穫される時に農地から持ち出される。この収奪された養分を農地に補給しなければ,土の肥沃度は低下する。化学肥料が世の中に登場した19世紀より遙か前の時代,堆肥は農地から収奪された養分の補給に用いる養分移転資材として考え出された(詳細は本連載の第11回(2022年5月号) 参照) 。
In this article, we will consider the challenges of labor and land to produce and actively use compost as a nutrient transfer material, which is especially necessary in organic farming.
1. paddy rice cultivation does not require large amounts of compost due to the high natural supply of nutrients.
The widespread and general use of chemical fertilizers in the world is a relatively new story, having begun after World War II. Before that, compost was the main source of nutrients. However, the dependence on compost as a source of nutrients is small in rice cultivation and large in field cultivation. Let us first look at the rice crop to see why.
Rice paddies are flooded with water. At this time, nutrients such as nitrogen and potassium dissolved in the water are naturally supplied to the paddy field together with the water. At the same time, the paddy field under waterlogged conditions is in a reduced state of oxygen deficiency. Phosphorus and iron, which were originally difficult for plants to absorb in the soil, are converted to easily absorbable forms when they are reduced and made effective. The paddy field system has a large supply of nutrients from nature. Therefore, even if nutrients are not actively provided, the fertility of the soil does not decrease significantly. Therefore, rice paddies do not require a large source of nutrients to maintain soil fertility. This is the reason for the low dependence on compost as a source of nutrients. This may be one of the reasons why organic farming is more feasible in paddy rice cropping.
Therefore, the raw materials for producing compost as a source of nutrients were wild grasses and weeds growing outside of farmland, as well as leaves, branches, undergrowth, paddy field weeds, and straw, all of which could be collected through diligent human labor. In Japan, the use of livestock manure for compost production was not common, and livestock were mainly used as service animals.

2. crop rotation is a prerequisite for field cultivation, and composting using livestock is a source of nutrients.
Field crops cannot be grown in rows like rice paddies, and crop rotation is a prerequisite for field cultivation. Furthermore, fields do not have a natural supply system of plant nutrients as rice paddies do. Therefore, if the fields are not supplied with nutrients, crop yields will drop dramatically. Therefore, before the advent of chemical fertilizers, farmers relied heavily on compost as a source of nutrients. The idea of actively using livestock in field crop areas, especially in Europe, was then conceived. The final result was the four-year crop rotation of the Norfolk method, which was perfected in England in the 19th century.
ノーフォーク農法では,家畜のエサとなる飼料作物(飼料用カブとアカクローバ)を栽培して土の中にある養分を吸収させ,次にそのエサを家畜に与えて,その家畜のふん尿という形態で養分を回収す。家畜は家畜舎で飼養するため,ふん尿の回収率も高い。最終的にそのふん尿を原料に堆肥を生産し,それを人の食料生産の畑(コムギとオオムギを栽培)へ与えて農場内での養分循環系を成立させた。堆肥という養分源を最大限に生かしたこの超集約的農法は,コムギ生産量をおよそ2倍にするほどの画期的な農法となった(詳細は前述した本連載第11回を参照) 。
3. the origin of the Norfolk farming method is the Flemish saying
The Flemish region of mainland Europe (present-day southern Netherlands to western Belgium and northern France), across the river from the birthplace of Norfolk agriculture, has an old saying: "No fodder, no livestock; no livestock, no fertilizer; no fertilizer, no harvest. This saying tells us that compost plays an important role in European crop rotation as a source of nutrients. This is the very starting point of the Norfolk farming method.
There is another important point to be made in this adage. That is, the production of compost as fertilizer requires feed for livestock. As the saying goes, compost cannot be produced as a source of nutrients without producing crops that feed livestock (forage crops), not human food. That is why Norfolk Farming allocated half of the land area on the farm to fodder crop production, and added fodder turnips and red clover to the crop rotation. This increase in forage production increased the number of livestock that could be kept, which in turn greatly increased the amount of manure produced. Thus, it became possible to increase the production of manure as a source of nutrients. The increased production of manure increased the amount of inputs to the farmland, and the amount of nutrients given to the farmers increased. The result was an almost doubling of wheat yields, as already mentioned.
To support this, however, it was necessary to allocate half of the farmland for nutrient source production, i.e., cultivation of forage crops for livestock feed. This was a major land use challenge for the Norfolk farming method, which uses nutrient cycling to maintain soil fertility and sustain high crop production. The land area for human food production is only half of the farmland.
4. land for producing livestock feed is required for compost production using livestock.
How to secure the source of nutrients is an important issue when producing crops on a certain area as organic farming in field crops. It is important to remember that even in field crops, if we try to maintain soil fertility through nutrient cycling using livestock, as in Norfolk farming, labor for livestock rearing and land for livestock feed production are required in the cycle system. The amount of feed produced on the land determines the number of livestock that can be kept on the farm. The number of animals determines the amount of manure produced, which in turn determines the amount of manure produced. As the Flemish saying goes, "Without feed there is no livestock, and without livestock there is no manure.

5. what to do about the increased labor burden of compost production
家畜を利用しない畑作であっても,有機農業が目指す養分循環型の作物生産では,養分源の堆肥は循環系外で生産されたものを持ち込むのではなく,自給すべき資材である。緑肥の利用や作物の収穫残渣を利用した堆肥生産で養分源を確保する必要がある。しかも使い勝手の良い完熟堆肥とするには,堆肥の切り返しなどの管理労力を必要とする。有機農業に取り組む農家が,その面積を縮小する最大の理由は労力がかかることだという(農水省,2022) 。除草の他に堆肥生産で増える労働負担をどうするかも大きな課題だ。
The "Green Food System Strategy" launched by the Ministry of Agriculture, Forestry and Fisheries in May 2021 aims to expand organic farming to 25% of all arable land, or 1 million hectares, by 2050. However, I wonder if they have given much thought to the production of organic fertilizers necessary to supply nutrients to this area. It is highly doubtful.
−第7章− 有機農業と慣行農業
第39回 慣行農業の養分源・化学肥料の課題
−原料の資源枯渇や生産のエネルギー問題−
令和7 (2025) 年 2/3月合併号 (第768号)
In the previous issue, we discussed the weakness of nutrient-recycling organic agriculture, which requires the preparation of farmland for the production of compost as a self-sufficient nutrient source. In this issue, we will discuss chemical fertilizers used as a source of nutrients in conventional agriculture.
Chemical fertilizers are produced from mined ores. As long as this process is repeated, resource depletion is certain to occur, just as it does with fossil fuels. This is because the mineral resources used as raw materials for fertilizers are non-renewable. In the following, I would like to outline the problem of resource depletion in the order of phosphorus, potassium, and nitrogen, and point out that even in conventional agriculture, there is a major weakness in the source of nutrients.
1.リン−産出国の寡占化で安定供給にも不安
Chemical fertilizers began with the production of superphosphate lime by treating animal bone meal with sulfuric acid. However, as demand for fertilizers increased, animal bones became scarce, and in Europe, not only animal bones but also the bones of soldiers who had fallen on the battlefield were used. It was not until the discovery of phosphate ore in Florida and Carolina in the late 19th century that the raw material for phosphorus fertilizer was replaced by ore.
現在のリン鉱石は地球上で大きく偏在している。2023年の世界のリン鉱石埋蔵量は740億トン(アメリカ地質調査所,2024) ,その68%がモロッコに埋蔵されている。リン鉱石の産出量は年間2億2千万トン。世界最大の産出国は中国で,世界産出量の41%を占める。この中国にモロッコ,アメリカの3カ国で全産出量の66%に達する。
ロシアのウクライナ侵略後,世界各国は食料安全保障への危機感から食料生産の作付面積と施肥量を増加させている。その結果,肥料全般の需要が高まった。この需要をリンの供給が満たせるのは2040年ころまでと推定され,それ以降は需要が供給を上回ると想定されている(Cordellら,2013) 。現状からの推定では,リン鉱石資源は早くて30年,長期に見積もっても300年で枯渇すると見込まれている。しかも,高品位リン鉱石の採掘はかなり進んでいることから,今後は低品位のリン鉱石を利用せざるを得なくなる。低品位リン鉱石では,採掘後のリンの抽出コストがかさみ,価格上昇が避けられない。
Low-grade phosphate ores often contain heavy metals such as cadmium and arsenic, and radioactive materials such as radium and thorium as impurities. Therefore, there is concern that mining may pollute the surrounding environment.

2.カリウム−森林消滅の危機を救う鉱石の発見
カリウムの原料は森林の燃焼灰だった。植物を燃やした灰に水を加えて混合し,その上澄み液からカリウムを取り出した(これをポタッシという) 。このカリウムを含むカリ鉱石が,ドイツ中部の岩塩層の深部で発見されたのが1860年だった。
Potassium was used more as a raw material for gunpowder than for fertilizer. In the early 19th century, during the Napoleonic Wars, France actively used potash for gunpowder production. If potash had not been discovered in the mid-19th century, the forests of Europe would have been destroyed as forests were cut down to produce the potash needed to manufacture gunpowder. The discovery of potash ore was indeed a great discovery that saved Europe's forests from extinction. It was not until 1840, when the importance of potassium as a fertilizer ingredient was recognized, that potash ore began to attract attention as a raw material for fertilizer.
現在のドイツのカリ鉱石産出量は,2023年の世界産出量3,900万トンのうちの7%,世界第5位である(アメリカ地質調査所,2024) 。現在は,世界のカリ鉱石産出量のうち33%をカナダが,17%をロシアが,15%を中国が,10%をベラルーシがそれぞれ産出し,ドイツの7%を含めると,この5カ国で世界の82%が産出されている。リン鉱石と同様,カリ鉱石も地球上で偏在している。
アメリカ地質調査所(2024)によれば,2023年のカリウム資源埋蔵量は,回収可能鉱石量で少なくとも110億トンである。カリウム(K2O)相当量としての資源埋蔵量でみると,産出国と同じ5カ国で世界の79%を占めている。この回収可能鉱石量を現在の鉱石産出量で採掘すると,耐用年数はおよそ280年である。しかし,先のリン鉱石の場合と同様に,採掘の経済性が次第に悪化することや,鉱石中のカリウム含有率などの問題で,産出効率の低下は避けられない。リン鉱石と同様,資源の枯渇は想定より早くやってくるだろう。
3.窒素−肥料原料は鉱物資源から空中窒素へ
窒素肥料の鉱物由来資材は,硝酸ナトリウムを主成分とするチリ硝石が最初で,火薬原料としての需要が先行した。1840年代にはペルーから窒素やリンを含むグアノが,ヨーロッパへ輸出された。天然資源であるチリ硝石やグアノの急速な需要の拡大は,早くも19世紀末に資源枯渇の心配を招いた。1898年,イギリス学術協会の会長になったクルックス(1832〜1919)はその就任演説で,「チリ硝石の鉱床は近い将来掘りつくされる。それゆえ,空気中に無限に存在する窒素を植物に利用できるように変え,肥料にすることが重大かつ緊急の課題である」と訴えた。
これに触発され,空気中の窒素ガスを工業的に肥料原料にする研究が進んだ。それを可能にしたのがドイツのアンモニア合成法,ハーバー・ボッシュ法だった。ハーバー(1868〜1934)はこの業績で1918年に,ボッシュ(1874〜1940)は高圧化学の業績で1931年にノーベル化学賞を受賞した。スミル(1943〜)は「20世紀最大の発明は,飛行機,原子力,宇宙飛行,テレビ,コンピュータではなく,アンモニア合成の工業化である。これなくして,20世紀に人口が16億から60億まで増加することはなかった」とまで指摘している(スミル,2001) 。
窒素は空気中の窒素ガスを原料とするため,原料の枯渇に不安要素がないと思われがちである。しかし,このアンモニア合成法にも課題がある。それは,この反応には高温高圧という条件があること,窒素ガスと反応させるための水素ガスは,重油,原油,コークスガス,天然ガス,ナフサなどに含まれる炭化水素を高温分解して製造されることなど,膨大なエネルギーを必要とすることである。このエネルギー消費は,全人類が消費するエネルギーの数%以上にもなるとの指摘がある(芦田ら,2022) 。それほどのエネルギーを消費するにも関わらず,アンモニア合成の回収率が30%程度と少ないことも問題である。ハーバー・ボッシュ法が有限資源の化石燃料をエネルギー源として使用する限り,アンモニア合成を持続的に続けることはできない。
最近,この問題を克服する成果が公表されている。芦田らが,常温常圧の温和な反応条件で可視光エネルギーを用いて空気中の窒素ガスからアンモニアを合成することに世界で初めて成功した(Ashidaら,2022) 。この反応はイリジウム光酸化還元触媒とモリブデン触媒を用いた触媒反応である。この合成反応の工業化に期待したい。もしそれが実現し,触媒物質の資源が十分なら窒素肥料の資源枯渇問題は克服できる可能性がある。
As we have seen, all nutrients provided to farmland are finite resources. Finding ways to reuse these nutrients without wasting them is an extremely important task for us as we face resource depletion.
連載のおわりに
第40回 わが国農業者の高齢化は食料生産への不安要因
−高齢化歯止めの鍵は新規参入者支援−
令和7 (2025) 年 4月号 (第769号)
This is the final article in this series. I would like to take a short break from the soil to discuss the possibility of Japanese agriculture becoming "senile" and the key to overcoming this situation is to support and promote the entry of young people into the agricultural industry.
1. less than 1% of the population is engaged in key agricultural activities
わが国は,高度経済成長時代(1955年から1973年ころまで)に人口が急増した(図1−上) 。その後,2010年まで人口増加が継続し1.28億人でピークとなった後,減少に転じた。ところが,日常的に農業を自営する人(基幹的農業従事者。以下では基幹農業者と略)は1960年以降,一方的に減少している。とくに人口急増の高度経済成長期に著しい。すなわち,1960年の基幹農業者は1,175万人(人口比12.6%)だったが,75年には489万人(人口比4.4%)にまで大きく減少した(図1−上) 。人口比でみると,75年は60年の実に3分の1にまで落ち込んだことになる。
It is clear that people moved from agriculture to industry during this period. At the same time, this period coincided with a sharp decline of more than 20% in Japan's food self-sufficiency ratio (on a calorie basis), from 79% in 1960 to 54% in 1975. This was the result of importing agricultural products and exporting industrial products to promote economic growth.
基幹農業者の減少傾向はその後も変わらず,2024年には1960年のわずか9%の111万人,人口比では1%を下回ってしまった(図1−上) 。
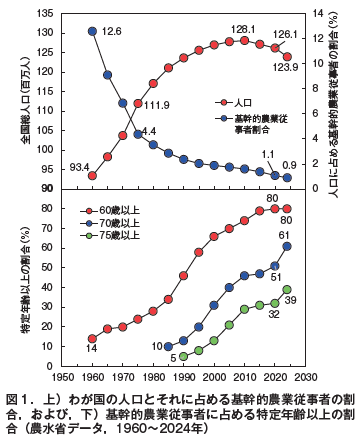
2. aging of key farmers
わが国は高齢化が加速している。基幹農業者も例外ではない。基幹農業者は減少と同時に高齢化が進み,基幹農業者に占める60歳以上の割合が,1960年には14%だったのに対し,2024年は80%に達している(図1−下) 。
とくに直近の2020年から24年の変化が興味深い。すなわち,60歳代以上の割合は20年以前と異なり,この4年間は増加が停滞した(図1−下) 。一方,この期間に割合が大きく高まったのが70歳以上で,75歳以上もそれ以前より高まっている。この事実は,60歳未満の基幹農業者が十分に補充されずに高齢化が進行したことを示している。これが単に一時的な現象なのか,この傾向が持続するかは注意深く見守る必要がある。
The average age of key farmers has increased consistently from 59.6 years in 1995, the year for which data are available, to 69.2 years in 2024. At this rate, there is a possibility that our country's agriculture industry will cease to function due to "senility" in less than 30 years.
3. the downward trend in the number of new farmers continues unabated
わが国農業の「老衰」を防ぐには,若年層の新規就農者を増やすことにつきる。しかし,新規就農者は2006年に81.0千人だったにも関わらず,23年には43.5千人にまで半減した(図2) 。ただし,基幹農業者も減少したため,基幹農業者に対する新規就農者の割合は,15年以降,3.8%内外で大きな変化はない(図2) 。
新規就農者は,以下の3区分からなる。すなわち,①新規自営農業就農者(個人経営の農家の世帯員で,調査前の1年間の生活が自営農業への従事を主とする者。以下,新規自営就農者と略) ,②新規雇用就農者(調査前1年間に新たに法人等で常雇いとして雇用され,農業に従事した者) ,③新規参入者(土地や資金を独自に調達し,調査前の1年間に新たに農業経営を開始した者)である。
新規就農者の大部分は新規自営就農者である(図2) 。新規自営就農者は親から継承した担い手とみなせる。新規自営就農者のうち50歳以上の割合は,2007年77%から23年の79%までほぼ変化していない(図3) 。基幹農業者の親は高齢化しながらも,子への経営移譲時期に大きな変化がなかったのだろう。そもそも基幹農業者自体が減少しており,それを継承する新規自営就農者に増加傾向はない(図2) 。しかも50歳以上が中心で,基幹農業者の高齢化の歯止めにならない。
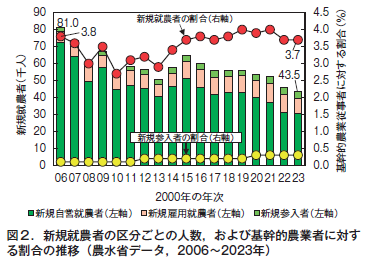
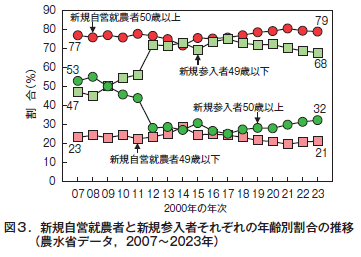
4. very few new entrants
一方,新規就農者のうち新規参入者は比較的若年層(49歳以下)の割合が多い(図3) 。新規参入者のうちの若年層割合は2012年以降,およそ70%内外で高止まりしている。したがって基幹農業者の高齢化を止めるためには,新規参入者の増加が必須である。しかし新規参入者は,2006年の2.2千人から23年に3.8千人になったに過ぎない(図2) 。これは基幹農業者のわずか0.1〜0.3%でしかない。これでは基幹農業者の高齢化を鈍化させることすらできないだろう。なぜ新規参入者が増えないのだろうか。
5.新規参入の障害−資金と農地の問題
There are young people from the city who are not familiar with agriculture, but who yearn to become farmers. I have come in contact with many such young people. However, when they started farming, they had to face difficulties in raising funds and acquiring farmland.
Various support measures (e.g., funds for starting business, funds for young farmers, and business development support programs) are available from the national and local governments. However, there are conditions for approval to receive these funds, and in reality, it is not easy to procure funds. It is necessary to ease the requirements for receiving these funds to make it easier for newcomers to obtain loans and subsidies.
農地は国民の食料生産の基盤で貴重な社会資本である。それゆえ,農地を適切に保全するために農地法が制定されている。農地を取得するには,取得のための要件を満たした上で,農業委員会の許可をもらう。実際の取得で大きな課題は,土地価格である。わが国の農地価格は欧米各国に比べ7〜30倍も高い(農水省,2010) 。
The young people who have passed through these difficulties and made their dreams come true are the ones who will lead the rejuvenation of our country's key farmers. Their support is extremely important.
Acknowledgements
I would like to express my sincere gratitude to all the readers who have enjoyed reading this series of articles over the past four years. I would also like to express my deepest gratitude to all those who carefully reviewed my manuscripts and to the editorial staff who encouraged me in my writing. Thank you very much.
著者略歴
松中 照夫(まつなか てるお)
1948年兵庫県尼崎市生まれ,酪農学園大学名誉教授。
1971年北海道大学卒業後,農学部助手(農芸化学科土壌学講座)を経て,北海道南根室地区農業改良普及所で農業改良普及員として活動。その後,1976年から,北海道立農業試験場にて,土と作物生産の研究に従事。この間1991年から92年イギリス・ノーフォークのモーレイ研究センターに留学。1995年から,酪農学園大学酪農学部酪農学科教授。土壌作物栄養学研究室にて,土と作物の栄養に関する教育と研究に従事。
2013年日本草地学会賞受賞,2014年酪農学園大学を定年退職し,名誉教授。
2014年から18年ホクレン農業協同組合連合会肥料農薬部特任技監,2018年から23年ジェイカムアグリ株式会社北海道支店技術顧問・ホクサン株式会社北広島工場顧問。