

Site Search
Search within product
No. 721 Published 2020 (R02) .06
Click here for PDF version
Agriculture and Science 2020/6


Fertilizers and nutrients:
Coated phosphorus ammonium nitrate (long, high control, Nutricote)
Characteristics of (Part 2)
Shibata Professional Engineers Office
Masaru Shibata
(6) For agriculture in harmony with the environment: the active role of fertilizer with regulated fertilizer effects
In view of the impact of agriculture on the environment, soil fertilizer technology has been compiled as the basis for harmonizing the environment and productivity. The simplification of soil diagnostic techniques and the practical application of software that calculates the appropriate amount of fertilizer based on the data collected have helped to optimize the use of fertilizers. In addition, the use of fertilizer with a high nutrient utilization rate and controlled fertilizer application is recommended as a measure to reduce the impact of nitrate-form chisso on water quality. In this context, coated fertilizers, which can supply nutrients in accordance with crop growth (nutrient absorption), have become well understood and are used for many crops as a typical fertilizer with regulated fertilizer use that harmonizes productivity and the environment (Figure 5).

(7) New fertilizer application technology brought about by coated fertilizers
Coated fertilizers are characterized by their ability to supply nutrients in proportion to the amount of nutrients absorbed by the crop and to reduce losses due to runoff, as well as by their well-controlled leaching, which makes it possible to apply fertilizers in a base fertilizer-intensive system. Coated fertilizers are base fertilizers in terms of application timing, but their function is that of a supplementary fertilizer. The coated fertilizers are applied together with the base fertilizer, which logically reduces the amount of additional fertilizer, and the base fertilizer-intensive fertilization method has taken root, harmonizing the environment and productivity.
The revolutionary fertilizer application method brought about by coated fertilizers is the root zone fertilization method. Conventional fertilizers are usually applied in a full layer or intentionally at a distance from the roots, because fertilizer components cause concentration disturbance to the roots and significantly inhibit growth when applied close to the roots. Coated fertilizers supply nutrients according to the amount absorbed by the crop, so they do not cause concentration disorders such as root scorching, and can be applied close to the roots, resulting in improved nutrient utilization and reduced fertilizer application. The nutrient for which harmony with the environment is most needed is the thioester component, and the advent of coated fertilizers has made it possible to optimize (reduce) the amount of thioester fertilizer applied (Figure 6).
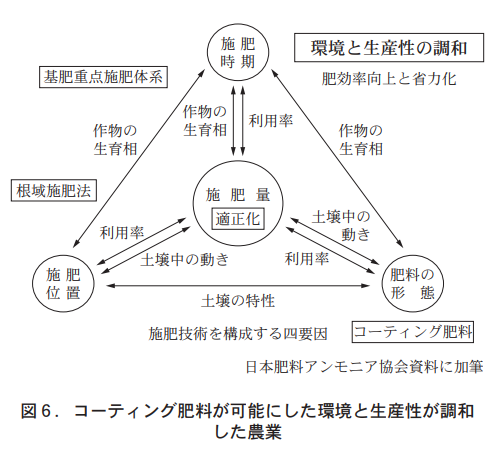
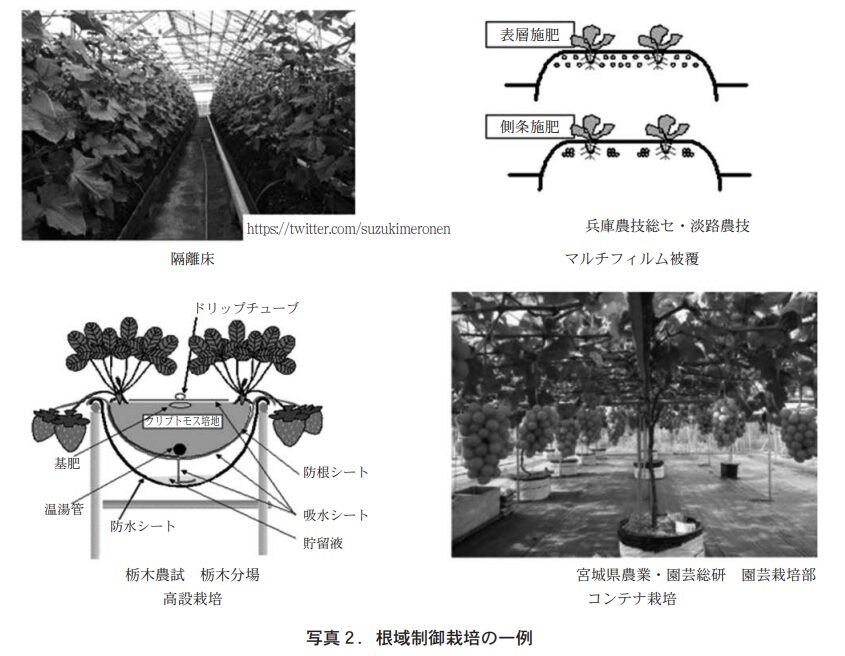
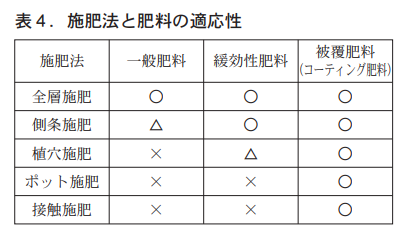
Table 4 summarizes the adaptability of fertilizer application methods and fertilizers. Fertilizer application location is closely related to nutrient utilization; the closer the fertilizer is to the roots, the higher the utilization, but on the other hand, there are concerns about root damage. Coated fertilizers with excellent leaching control performance enable local application of fertilizers in planting holes, in pots, and by contact, which could not be achieved with synthetic slow-release fertilizers.
Coated fertilizers can also be defined as fertilizers that can significantly increase the amount of fertilizer applied relative to the soil volume. Polyolefin resin-coated fertilizers, in particular, have high leaching control performance and do not cause root scorch, enabling the practical application of these new fertilization methods. The cultivation method using localized fertilization, which has developed in place of the full-layer fertilization method, can be called root-area controlled cultivation. The total box fertilization of rice seedlings using LP coat, coated fertilizers, and "seedling box leave" is the ultimate example of such a method. In the horticultural field, various root zone control methods are actually used, such as isolated bed cultivation in greenhouses, mulch film-covered cultivation, elevated strawberry cultivation, and container cultivation of fruit trees, and the use of coated fertilizers is expanding.
One thing to keep in mind when using root zone control cultivation with coated fertilizers is that since the amount of soil per fertilizer is reduced, it is necessary to consider how to deal with the reduced nitrification capacity of the soil. In the case of cultivation methods using artificial media such as coconut fiber or rockwool, the nitrification capacity is even smaller than in soil cultivation. Particularly, when coated urea is applied to the root zone, the nitrification potential of the root zone should be taken into consideration. When cultivating horticultural crops with a high amount of nitrogen fertilizer in the root zone, it is preferable to use a coated fertilizer containing nitrate-form nitrogen that is supplied continuously and systematically. It would be a waste if the roots of the crop are damaged.
(8) Characteristics of coated fertilizers containing nitrate-form chisso
Most of the brands of coated compound fertilizers in Japan and around the world are based on NPK compound fertilizers containing nitrate-form chisso, and the distribution of coated compound fertilizers based on ammonia-based compound fertilizers is unheard of.
Ammonium nitrate is a water-soluble fertilizer containing 351 TP3T of water-soluble chisso (17.51 TP3T each of ammonia- and nitrate-form chisso), and is considered to be a raw material for coating fertilizer as well as urea containing 461 TP3T of water-soluble chisso, but the risk of explosion increases when ammonium nitrate and organic matter coexist, making ammonium nitrate However, the coexistence of ammonium nitrate and organic matter increases the risk of explosion, making ammonium nitrate alone inappropriate. The addition of phosphoric acid and potassium eliminates this risk, so the granular raw material for coated fertilizers containing nitrate-form nitrate is a compound fertilizer with a nitrate complex NPK composition. Of course, the safety of each brand of coated fertilizer products has also been confirmed.
The most important characteristic of the nitrate-form nitrate-containing coating composites is shown by the "root response to fertilizer" (Photo 3). Photo 3 shows the results of a test in which 5 g of nitrate-form fertilizer was placed at the bottom of a 3 cm × 12 cm dia. paper cylinder pile sealed with fertilizer-free soil was inserted into a root box in which tomato seedlings were planted. The fertilizers used were "Kumiai Phosphonitri Kari S555," "Long 424-70 Type" (coated phosphonitri Kari), and "LP Coat 70 Type" (coated urea).
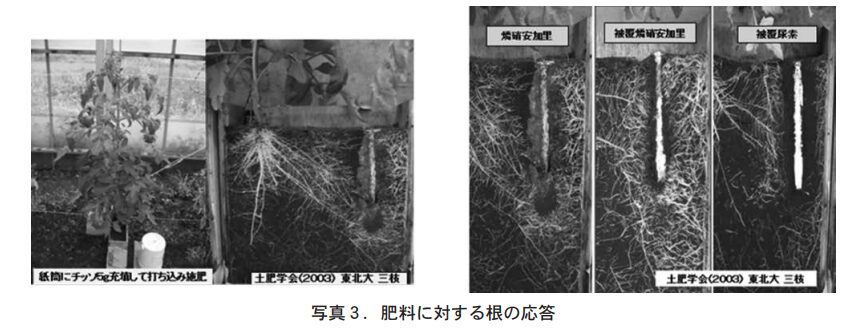
As shown in Photo 3 (left), plant roots are thought to grow by sensing the presence of nutrients through EC (electrical conductivity), but root extension is affected by the type and concentration of nutrients present in the soil. As shown in the right side of Photo 3, in the phosphonitrite-alkali area, the nutrient concentration is high just below the paper tube, so the roots spread out as if they were receiving nutrients at a little distance from the bottom of the paper tube. In the urea-phosphorus-coated long 424-70 type, the amount of roots around the paper tube was high, and white roots were surrounding the paper tube as if they were swarming around it. The inactive root elongation in the urea-coated zone is assumed to be due to a failure of nitrification of ammonia produced by the decomposition of urea, which is caused by the accumulation of ammonia. Caution should be exercised when using coated urea in root zone controlled cultivation of horticultural crops with high fertilizer application.
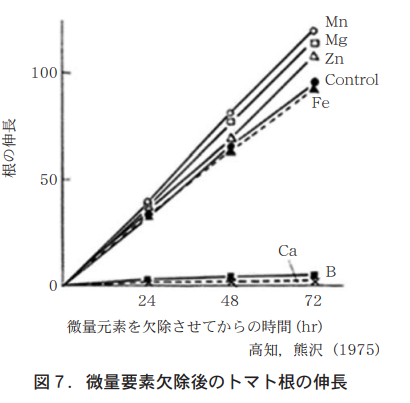
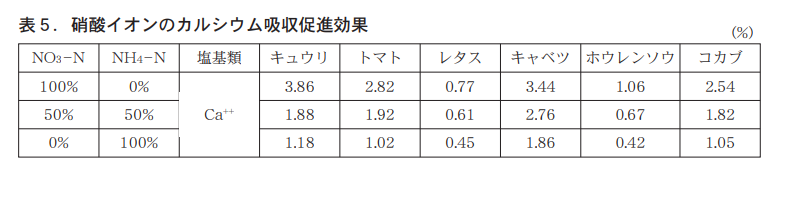
It is known that the absence of calcium (also boron) dramatically arrests root elongation (Figure 7). Calcium (also boron) plays an important role in the formation of cell walls, and calcium absorption is essential for root elongation. Nitrate-form chisso is known to promote calcium absorption (Table 5), and the superior root elongation in the long zone is presumably the result of calcium absorption promotion. Long is also a fertilizer that promotes healthy root growth.
(9) Significance of covering fertilizers with nitrate-form chisso
Nitrate-form chisso is an essential nutrient for horticultural crops, but nitrate ions are not easily adsorbed by the soil, so they are easily transported to the subsoil layer of the field through subsurface infiltration of rainwater. The significance of nitrate-form nitrate cover is to increase the efficiency of nitrate-form nitrate utilization by continuously absorbing nitrate-form nitrate, which is an important nutrient for crops. The significance of nitrate-form chisso coverage is summarized in Table 6.

Soil and fertilizer management to improve soil fertility in tea plantation soils
Jcam Agri Corporation Kyushu Branch
郡 司 掛 則 昭
Introduction
Tea production in Kagoshima Prefecture is thriving, with tea plantation area of 7,990 ha and rough tea production of 28,100 t in 2018, both second in Japan. However, the current state of soil fertility in tea plantation soils has been pointed out to have problems mainly in chemistry, such as low pH, accumulation of phosphoric acid and potassium, and nutrient imbalance.
Low pH is particularly serious. Tea is a crop that prefers a low soil pH, and low pH itself is not so much of a problem. However, it has been reported that tea growth and yield are affected when pH 4 or lower is strongly acidic, because the amount of fine roots that absorb nutrients in the tea plant decreases and its fertilizer absorption capacity decreases (Nonaka, 2005). On the other hand, as a result of the long-standing practice of high-fertilizer cultivation in tea plantations for high yield and quality improvement, the accumulation of fertilized phosphoric acid and potassium in the soil tends to become apparent, and improved fertilizer application is required to reduce the accumulation.
Here, as a continuation of the article "Actual Soil Fitness and Soil Preparation of Kagoshima Prefecture Tea Plantation Soils" published in the May 2017 issue of the same journal, we would like to discuss specific measures for soil and fertilizer management to solve soil fertility problems such as low pH, phosphoric acid and potassium accumulation that tea plantation soils are facing.
2. current status of soil fertility in tea plantation soils and countermeasures
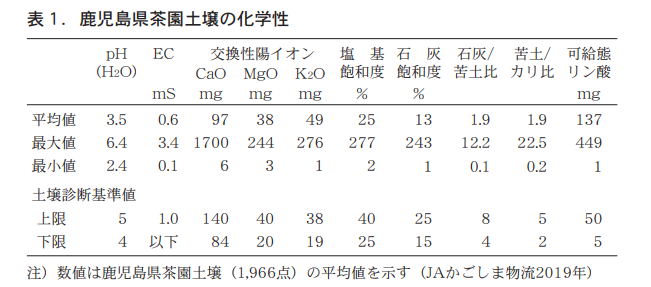
According to recent soil diagnostic results on Kagoshima Prefecture tea garden soils (Nagata, 2019), pH averaged 3.5, well below the diagnostic standard value of 4 to 5 (Table 1).
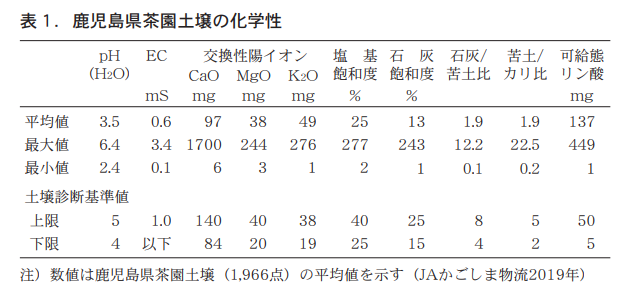
EC averaged 0.6 mS/cm, exchangeable calcium and magnesium averaged 97 mg and 38 mg, respectively, which were within the appropriate range of soil diagnostic criteria, while exchangeable potassium averaged 49 mg, which was higher than the diagnostic criteria. Base saturation and lime saturation were 25% and 13%, respectively, which were almost equal to or less than the lower limit of the diagnostic criteria, while lime/bittern ratio and bittern/potassium ratio, which indicate nutrient balance, were both 1.9, which were below the standard values. The lime/bituminous soil ratio was particularly high. The average value of dietary phosphoric acid was 137 mg, more than twice the upper limit of the diagnostic criteria (50 mg).
Thus, the current state of soil fertility in tea plantations is mainly due to chemical problems such as low pH, phosphate and potassium accumulation, and nutrient imbalance. The most effective countermeasures are acidity correction by applying calcareous materials to raise pH and reducing fertilizer to prevent the accumulation of phosphate and potassium nutrients. The nutrient imbalance can be corrected by correcting the acidity and reducing the amount of fertilizer.
3. factors that cause soil pH to decrease and acidity to correct
(1) Factors that decrease pH
One of the reasons for the decrease in soil pH is that the tea plantation soils are black granite soil. Black granite soil is composed of volcanic ash, which contains a large amount of active aluminum, which is hydrolyzed to release hydrogen ions and acidify the soil. However, this aluminum hydrolysis reaction alone does not reduce the pH below 4. In addition, other nutrient conditions, such as low exchangeable calcium and high concentrations of soluble phosphate, are thought to be deeply involved in lowering the pH.
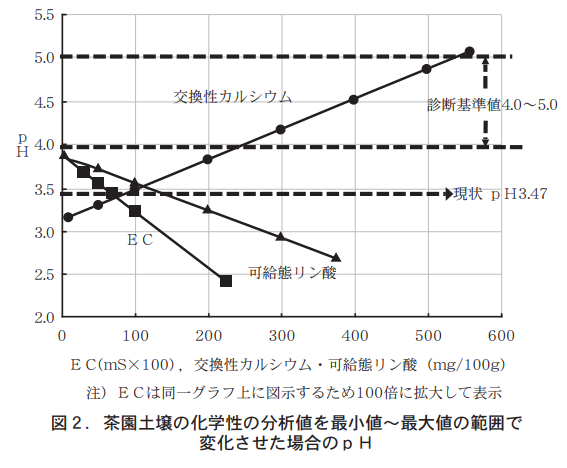
A graphical representation of the relationship between pH and other chemical properties for the 436 Kagoshima Prefecture tea garden soils introduced in the May 2017 issue of the same journal shows that pH decreases as exchangeable potassium and salable phosphate increase, but conversely increases for exchangeable calcium or magnesium (Figure 1). However, since it is difficult to determine the percentage contribution of each chemical property to the increase or decrease in pH from the graph, we performed a multiple regression analysis with pH as the objective variable and the other chemical properties as explanatory variables, resulting in the following multiple regression equation.
pH = -0.62*** x EC + 0.0032*** x exchangeable calcium - 0.0032*** x ratable phosphate + 4.05
Here the coefficient of determination R2 = 0.80, and an asterisk (**) in each partial regression coefficient indicates that it is statistically significant at the 11-TP3T level.
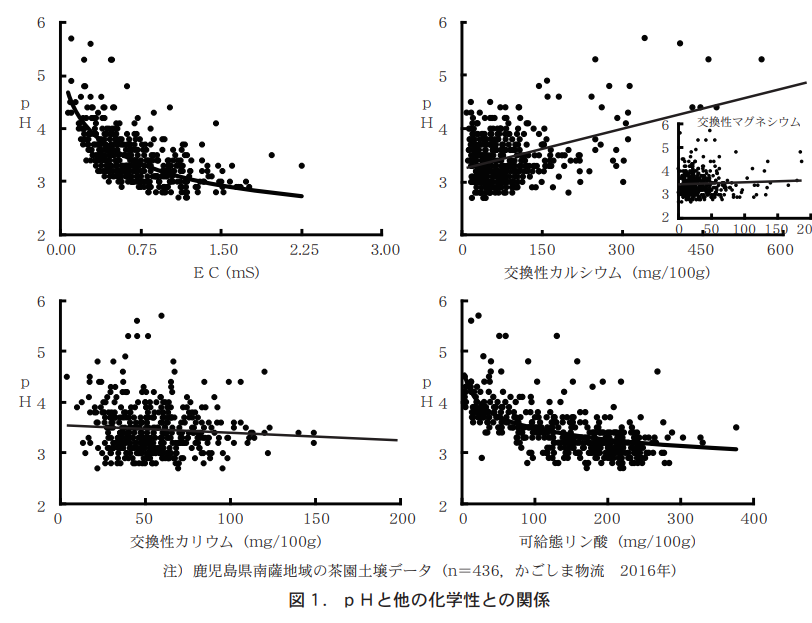
From this multiple regression equation, it is clear that EC, exchangeable calcium, and free-form phosphate are the chemistries that contribute significantly to pH change. Furthermore, when the analytical values of these three chemistries varied within the range of minimum to maximum values (Table 1, Fig. 2), the pH did not reach the lower limit of the diagnostic standard value, 4.0, even when the analytical values of EC and exchangeable phosphate reached the minimum, but increased to 4.0, which is within the diagnostic standard value, when the amount of exchangeable calcium increased to about 250 mg However, when exchangeable calcium is increased to about 250 mg, the pH level rises to 4.0, which is within the diagnostic standard. Therefore, it can be inferred that increasing exchangeable calcium is the simplest and most effective method of acidity correction.
(2) Target pH for acidity correction
(2002) reported that the target pH for acidity correction is 4.5 because the retention of ammonia nitrogen, which is important for tea tree growth, is unexpectedly low in the pH range of 4 to 4.4 and is easily leached out. Based on this, the target pH was set at 4.5. Based on this, the amount of calcareous material (calcium carbonate) required when the target pH is set at 4.5 was determined by the following procedure using multiple regression equations.
(1) Find the exchangeable calcium content at the current pH of 3.47 from the multiple regression equation (= 87 mg)
(ii) Find the exchangeable calcium content at target pH 4.50 from multiple regression equation (= 418 mg)
(3) Subtract (1) from (2) to obtain the amount of exchangeable calcium required for acidity correction (= 331 mg)
(iv) Convert the amount of exchangeable calcium into the amount of calcareous material applied (charcoal calcium) (= 591 mg)
Here, the average values of soil depth and provisional specific gravity of Kagoshima Prefecture tea garden soils are 13 cm and 0.66, respectively (Kyushu Agricultural Administration Bureau, 2010), and if the ridge area is 1/5 of the tea garden area, this 591 mg is equivalent to about 100 kg of calcium carbonate per 10 a. The average values of soil depth and provisional specific gravity of the Kagoshima Prefecture tea garden soils are 13 cm and 0.66, respectively (Kyushu Agricultural Administration Bureau, 2010).
The amount of calcium carbonate applied at about 100 kg/10a is almost equivalent to the amount of calcareous materials commonly applied for soil management in black box tea plantations, and the estimation of the amount of calcareous materials applied by the multiple regression equation here seems reasonable.
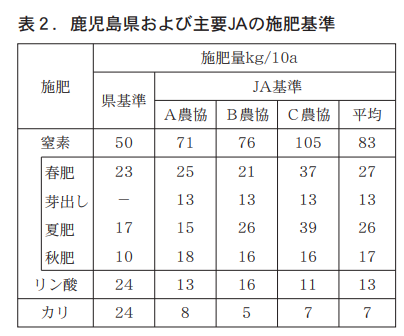
4. improved fertilizer application to prevent nutrient accumulation
In the section 2, "Current status of soil fertility in tea plantations," it was pointed out that phosphoric acid and potassium are accumulated in tea plantations, and this is well recognized not only by the prefecture but also by JA and other organizations. However, it is not clear whether these reduced fertilizer standards provide sufficient nutrients for tea plant growth, so we examined the annual nutrient balance of phosphoric acid and potassium to verify the validity of the current standards.
1) Annual nutrient budget for phosphate and potash
It is not easy to accurately measure the nutrient balance of phosphate or potassium by tea plants, but it is possible to estimate the balance of phosphate or potassium based on a nitrogen balance model (Kagoshima Agricultural Research Center 2006). The estimation method is based on the nitrogen balance model, which shows the amount of fertilizer nitrogen absorbed by tea leaves and leaves with trimmed branches and the distribution ratio to the nutrient concentration in the soil, and then divides the phosphoric acid and potassium by the estimated amount. Assumptions are: 1) the utilization rate of fertilized phosphoric acid and potassium is 20% and 50%, respectively; 2) soil concentrations of phosphoric acid and potassium are converted to kg/10a by multiplying the analytical value of mg/100g by a provisional specific gravity of 0.75; 3) phosphoric acid leaching is assumed to be zero; and 4) potash leaching is estimated from the amount of potash leached from crop soil in a black box field. The amount of potassium leaching was estimated from the amount of potassium leached from the crop soil in the black bok field (Noguchi, 2015).
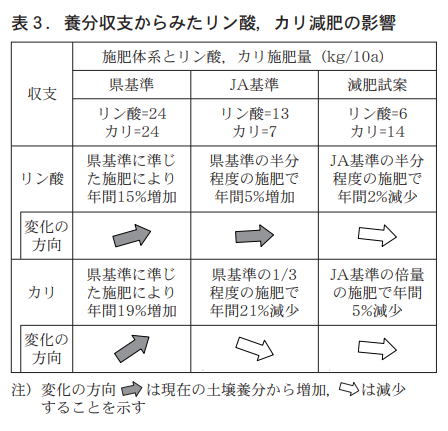
According to the annual balance of phosphoric acid and potassium determined in this way, if the prefectural fertilizer standard is followed (Figure 3), a fertilizer application of 24 kg of phosphoric acid would result in a balance of +16 kg of phosphoric acid and an estimated increase of 151 TP3T per year from the current 104 kg/10a to 120 kg/10a of soluble phosphoric acid. On the other hand, the annual balance of potash will be +8 kg, and exchangeable potassium, which is currently 42 kg/10a, will increase by 191 TP3T to 50 kg/10a. Therefore, fertilization according to the prefectural standard for both phosphate and potassium will increase the current concentration of nutrients in the soil and cannot be used as a measure to prevent accumulation.
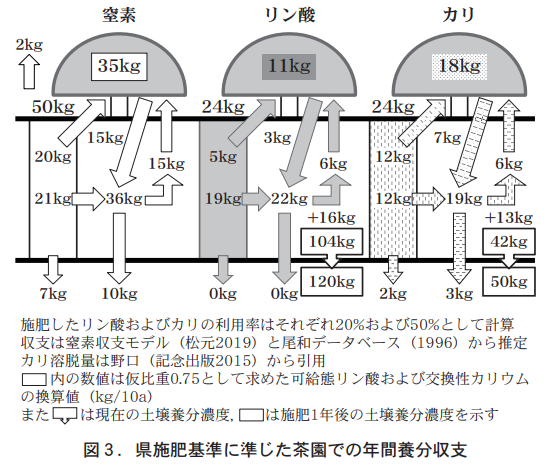
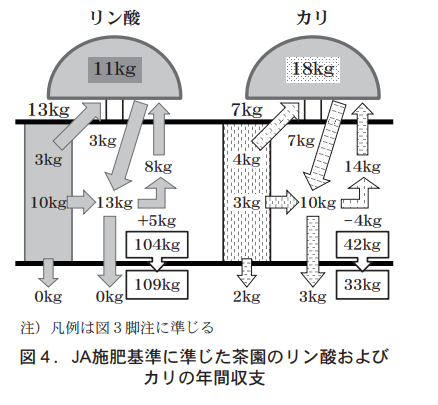
In contrast, if the JA standard is followed (Fig. 4), the annual balance with 13 kg of phosphoric acid fertilizer is +5 kg, and the annual increase in edible phosphoric acid is 51 TP3T from 104 kg/10a to 109 kg/10a, so it is assumed that further fertilizer reduction is necessary because the current JA standard cannot prevent accumulation.
On the other hand, the balance of potassium is -4 kg after 7 kg of fertilizer, which is the JA standard, and -9 kg after adding leaching, resulting in an annual balance decrease of 211 TP3T, which can prevent potassium accumulation. However, potassium decreases as much as 211 TP3T per year, and if this process is repeated, potassium is estimated to be below the soil diagnostic standard after 2 or 3 years. Therefore, it is recommended that the potassium level be set higher than the current fertilizer standard.
(2) Proposed changes to fertilizer reduction standards
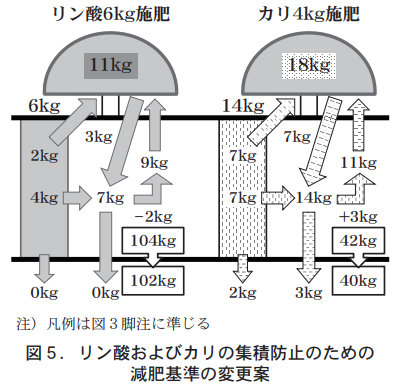
As summarized in Table 3, the annual nutrient balance of phosphoric acid and potassium suggests that fertilization with phosphoric acid according to the current JA standards would not avoid accumulation due to an increase in ratable phosphoric acid, and that fertilization with potassium would cause a rapid decrease in exchangeable potassium and could lead to a drop below the diagnostic standard. For these reasons, it is necessary to change the current standards, and to prevent accumulation of phosphoric acid and potassium while slowly decreasing the amount of accumulated nutrients, we propose a change to 6 kg of phosphoric acid, which is roughly half the current standard, and a change to 14 kg of potassium, which is twice the current standard.
The annual balance of phosphoric acid and potassium in this proposal (Fig. 5, Table 3) is -2 kg and -2 kg, respectively, even if 5 kg of leaching is taken into account, and the concentrations of both phosphoric acid and potassium in the soil decrease slowly to prevent accumulation. Therefore, it seems to be effective as a proposal to reduce phosphate and potassium fertilizers.
Summary
The current state of soil chemistry in Kagoshima Prefecture tea gardens is characterized by extremely low pH, soil accumulation of phosphate and potassium, and nutrient imbalance, suggesting the need for soil and fertilizer management that can quickly resolve these soil fertility problems.
Among these, a significant decrease in pH affects nitrogen absorption and leaching, which are the most important factors for tea plant growth and quality, so optimization of pH is an urgent issue. Therefore, multiple regression analysis of tea garden soil chemistry has shown that exchangeable calcium, EC, and edible phosphate contribute significantly to pH fluctuations, and that soil management that increases exchangeable calcium is the most effective way to raise pH. It was inferred that the application of about 100 kg/10a of calcareous material (calcium carbonate) per year to correct acidity is appropriate.
It is difficult to prevent soil accumulation of phosphoric acid and potassium with the current JA standards, and in particular, a rapid decline in potassium was observed, so it was decided that the current standards should be changed. The proposed changes include increasing phosphoric acid fertilizer to 6 kg/10a, about half the current standard, and doubling the amount of potash to 14 kg, which would slowly prevent accumulation. It will be necessary to conduct a field demonstration to verify the validity of the proposed changes.
References
Owa, Naoto, Nutrient balance of agricultural products in Japan, 1996.
Kagoshima Prefectural Agricultural Development Center, Research Results for Extension, 2006
Kyushu Agricultural Administration Bureau, Changes in Agricultural Soils in the Kyushu-Okinawa Region, 2010
Noriaki Gunjikake, Agriculture and Science, 5, 2017
Shigeho Nagata, Materials for Zen-Noh Tea Fertilizer Study Group, 2019
Sumitaka Noguchi, A Collection of Questions and Answers on Soil and Fertilizers, 2016
Kunihiko Nonaka, Chakken-Hoho, No.100, 2005
Jun Matsumoto, Zen-Noh Tea Fertilizer Research Group Materials, 2019
Matsumoto, Jun et al., Dohi Journal, 73, 2002