

Site Search
Search within product
第750号 2023(R05).05発行
Click here for PDF version
§メロンとキュウリのマグネシウム,カリウムの欠乏症状と再移動
Former Graduate School of Natural Science and Technology, Okayama University
桝田 正治
No § Soil - No.21
「土は生きている」といわれるのはなぜ?
-土は生き物なのか
前 ジェイカムアグリ株式会社
北海道支店 技術顧問
松中 照夫
メロンとキュウリのマグネシウム,カリウムの
欠乏症状と再移動
Former Graduate School of Natural Science and Technology, Okayama University
桝田 正治
Introduction.
野菜の生理障害について,筆者は発生部位と生育段階ならびにその発生要因を一つの表にまとめて示した7)。それから約20年経った現在においても,それらの症状の要因に新しい知見は加えられていない。
このことは近年,野菜の生理障害があまり大きな問題となるには至っていないことを示唆する。種子繁殖性の野菜では,新品種の生育特性や連作に伴う病害の研究が多く生理学的研究そのものが少なくなったように感じる。栄養繁殖性のサツマイモ,サトイモ,ジャガイモ,ニンニク,イチゴなどはウイルスフリー原種を保存し,それから増殖させ種苗として市販されるが,通常,栽培者が1,2年繁殖していると新しくウイルスに感染してしまい,外見上の症状からは,病的あるいは生理的としてその要因を見分けることが難しくなる。勿論,マグネシウム,カルシウムの欠乏や拮抗阻害ならびに微量要素の欠乏によるものも少なくない。
Mg欠乏症は微量の亜硝酸ガスやアンモニアガスによって引き起こされる障害にも時には類似すること,さらには光化学オキシダントやハダニの害と酷似するものも多く,その要因をすぐには判断しにくい場合が多い。
The former gas disorder tends to occur in greenhouse cultivation, especially with the heavy use of organic fertilizers such as oil cake and chicken manure. In addition, the gas disorder is also more likely to occur in open field cultivation of direct-seeded vegetables such as spinach, radish, and komatsuna, because their leaves are in contact with the ground surface. The mechanism of gas generation has already been clarified, and it is necessary to control the acidity of soil pH and the amount of nitrogen fertilizers, especially ammonia-form nitrogen fertilizers, to suppress gasification.
後者については,図1の①~③に示したように,光化学オキシダント障害,強光や高温障害などがあげられる。これらは一般に知られる写真④のメロンの葉脈間クロロシスとも類似していることがある。このメロン着果節葉に発生する症状とMg欠乏との関連性を本稿では論じようとするものである。光化学オキシダントは工場や自動車などから大気中に排出される窒素酸化物(NOx-ノックスと読む)や揮発性有機化合物が紫外線を受けて生成される物質(オゾン(O3)やパーオキシ・アセチル・ナイトレート(PAN)など)が動植物の細胞に障害をもたらす。

The morning glory shown in the photo (1) in Fig. 1 is a typical example of such a disturbance, and was an indicator plant for photochemical smog in the 1970s, which the author also investigated at a farm affiliated with Kyoto University about 50 years ago. At that time, I learned that taros were sensitive to photochemical smog among vegetables. Even recently, when cucumber leaves temporarily encounter intense light in early summer, the veins of the leaves become mottled and whitened on the following two days. This phenomenon is probably caused by the inability of the reactive oxygen species (ROS) scavenging system to instantaneously process the excess light energy, but no evidence has been obtained.
In the Earls melon shown in Photo (2), high temperatures and temporary intense light also cause overnight discoloration of some of the spaces between the veins of the leaves. Although the earl melon is an extremely high temperature tolerant crop (no damage occurs on the leaves on the opposite side of the strong light even at 43°C), the high temperature combined with strong light causes damage to the leaves.
Unlike nitrite, ammonia gas, photochemical oxidants, or high light damage, the mite damage shown in photo (3) is characterized by blurred leaf symptoms, and as the disease progresses, many mites can be seen on the underside of the leaves with the naked eye. However, the initial symptoms are similar to those of Mg deficiency and are difficult to distinguish. In particular, it is better to suspect mite damage first rather than physiological disorders in vegetables at the beginning of the hot season.
Mg deficiency symptoms and component migration in melon defoliation and cucumber
(1) Overview of melon defoliation
一般にウリ科作物では生育中期以降,特に果実の肥大が進む頃になると近傍の葉の葉脈間にクロロシスを発現することが多い。1980年代には各地でプリンスメロンの葉枯れ症が多発し多くの試験場での研究報告はMg欠乏によるものとした。そんな中で兵庫県農業総合センターの津高12)は葉枯れ症がK欠乏によって生じていると報じた。筆者の知る限りではK欠乏とした論文は後にも先にもこれが唯一である。
藤本2)はハウスメロンの葉位別Mg含有率を正常株と葉枯れ症株について調査し,図2の結果を得ている。果実は第13節~16節に発生した側枝に着果させ,下位葉から上位葉まで主茎節位の葉分析を行ったもので,13節~16節のMg含有率は正常株では0.3%以上,発生株では0.2%以下となっていることが分かる。この結果から,同氏は葉枯れ症の発現レベルは,葉中Mg含有率で0.2%レベルにあるとし,葉枯れ症は果実の肥大成熟に伴って葉のマグネシウムが果実へ転流し,その結果,葉の葉脈間が黄化し,やがて黄褐色を呈して枯れるが,このタイプのものは成熟の目安とされることもあるほどで収量,品質にほとんど影響しないと述べている。
筆者4)も自根キュウリの生理障害,褐色小班葉が0.19%以下で発生すること,欠乏症状を呈した葉のMg含有率は正常葉のそれに比べて確かに低いことを認めている。しかし,これらの結果は,葉から果実へマグネシウムが移行することを示すものではない。従来,マグネシウムは移動しやすい成分とされているが3,10,13),本当に移動しやすい成分なのであろうか?
(2) Difficulty of component transfer within the cucumber tree
First, the author compared the changes in the components of hydroponic cucumbers, especially magnesium, with those of other components. The author replaced the culture medium of cucumbers (main branches at 22 nodes and lateral branches at 3 nodes) grown in the standard culture medium with tap water, and investigated the dry matter weight and content rate of leaves and roots at the 5th node over time. Nitrogen and phosphorus decreased significantly in the dry matter content of the roots (61% after 4 days of water treatment), and potassium and magnesium decreased further (74% and 82% after 8 days of treatment).
一方,葉においても窒素,リン,カリウムは大きく減少した。特に,リンとカリウムの減少率が著しく,それぞれ処理8日後には当初の60%,63%となった。これに対して,カルシウムとマグネシウムは,わずかに減少するに過ぎなかった5)。勿論,処理期間中に光合成により乾物重は増加する。その時の乾物率から葉における含有量を求め処理開始時を基準に相対比として示したのが図3である。
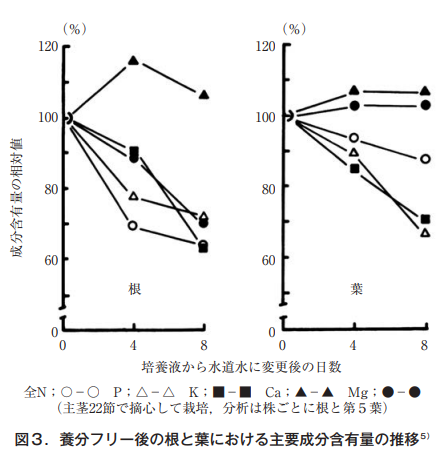
In the roots, calcium did not change at all, and the rate of decrease was total N=K>P=Mg>Ca. On the other hand, in leaves, calcium and magnesium did not change at all, and the rate of decrease was P=K>total N>Mg=Ca. The fact that there was no change in magnesium, which is considered to move frequently, indicates that magnesium in cucumber leaves is a less mobile element than nitrogen, phosphorus, and potassium, at least in comparison to nitrogen, phosphorus, and potassium, considering that the nutrient removal from the solution (tap water) was short, only 8 days.
(3) Magnesium sprayed on individual cucumber leaves does not move even on those leaves.
次に,生育中のキュウリを摘心し2本仕立てとし,草丈約70㎝に達した段階で培養液からマグネシウムを除き,それから2日毎に4回,2本仕立ての一方に1%MgSO4,Mg(NO3)2,あるいはMgCl2の溶液を樹全体に散布したところ,いずれの処理でも葉脈間クロロシスは完全に抑制できた(対照区:無散布の樹では発現する)。この散布効果が葉の表裏で変わらないことから,散布Mgはクチクラから細胞内に入り,そこで種々の形態に変化するものと考えられる。
The sprayed leaves themselves remained green, but the leaves of the upper and lower nodes showed chlorosis (photo omitted). This indicates how difficult it is for the sprayed magnesium to move.
そこで更に,個葉におけるMg移動の難易を知るため,クロロシスを発症すると予想される中位葉1枚を特定し,底を開けた紙コップを葉の一部に押し当てその中に1%MgSO4溶液を1日おきに4回散布した。その結果を図4に示す。
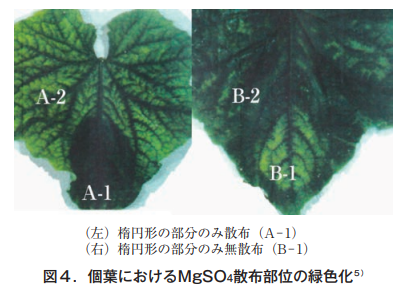
Sprayed areas remained green, while unsprayed areas exhibited typical intervein chlorosis. The Mg content in these sites is shown in Table 1.
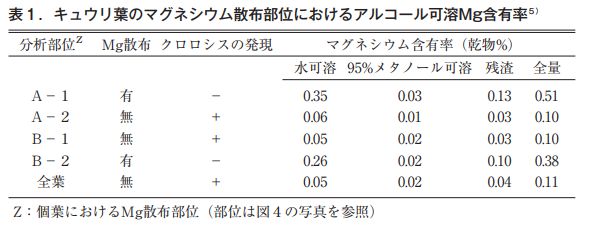
It is clear that the water-soluble Mg in sprayed sites A-1 and B-2 is about 6 times higher than that in unsprayed sites A-2 and B-1, and the content rate of the unsprayed site is the same as that of the whole-leaf unsprayed site, which was established as a control, so at least the sprayed Mg is not mobile in the individual leaves.
Despite the difference in leaf color between the sprayed and unsprayed sites, there was no difference in the magnesium in the chlorophyll backbone, i.e., 95% methanol-soluble Mg. This suggests that the amount of magnesium constituting chlorophyll is too small to be measured by ordinary instruments.
(4) White and green ring leaves of cucumber are induced by ammonia-form nitrogen.
接ぎ木キュウリ〈台木はカボチャ〉の白変葉が,1970年代後半から高知,宮崎で多発し十数年にわたり多くの研究者がその原因の究明にかかわった。当初,この症状はMg欠乏によるとする報告が多く発表されたが,これは,いわゆる黄化(クロロシス)ではなく白色壊死(ネクロシス)することから,Mg欠乏だけでは説明できない現象であった。多くは葉縁が緑色に保たれていることから’グリーンリング葉’と称され,激しい時は一夜のうちに褐変,光が当たると白変が形成される。このような葉の発生要因を,高知県園芸試験場の松本ら8)は,見事に解明したのである。簡単に要約すれば以下のようになる。
At that time, methyl bromide was used as a soil disinfectant in greenhouse cultivation. Oil cake, an organic fertilizer, was applied to the soil. The oil cake is converted to ammonia-form nitrogen by the action of microorganisms (ammonia-forming bacteria). Ammonia nitrogen is converted to nitrate nitrogen by nitrate-forming bacteria, and under low soil temperatures, the activity of the nitrate-forming bacteria is low (it is generally known that nitrate-forming bacteria are less sensitive to low temperatures than ammonia-forming bacteria), so the concentration of ammonia nitrogen increases. Furthermore, in soils treated with methyl bromide, the activity of nitrate-forming bacteria is weakened and the accumulation of ammonia nitrogen becomes more pronounced. Even if magnesium is present in the soil, its absorption is inhibited by antagonism when ammonia nitrogen is high. The greening of cucumber leaves is caused by an excess of ammonia nitrogen in the soil and inhibition of magnesium absorption.
Nevertheless, the author still felt that it was necessary to reproduce the symptoms observed in farmers' greenhouses in order to prove the factors that induce white discoloration of cucumber leaves, so he repeated trial and error in hydroponic cultivation of self-rooted cucumber and grafted cucumber. As a result, we found that white discoloration and green ring leaves appeared on cucumbers on pumpkin rootstocks when the concentration of ammonia nitrogen and magnesium in the nutrient solution were increased and decreased, respectively (Figure 5).

The technical term "interveinal necroticleaves" was first introduced in the revised Glossary of the Horticultural Society of Japan (2004), and green ring leaves were defined as a part of interveinal necroticleaves.
5)メロンの着果に伴う葉の障害とMg・K含有率の変動-マグネシウムが再移動するのは確かであろうか?
一般に,メロンは収穫期に近づくと果実近傍の葉にクロロシスが生じる。これが収穫期の目安として古くから語られてきた。教科書的にはこの症状は葉から果実へマグネシウムが移行するためであると記載されている。筆者も,大学の講義でそのように習ったような気がしている。アールスフェボリットでは,着果後40日ころから葉脈間がわずかに黄化しはじめ,50日頃には激しくなり褐変し始めることが多い。はたしてこれは,葉から果実へマグネシウムが移行することによって発現する症状であろうか?
そこで,果実の有無と葉の障害程度を調べるため,果実を着果後40,50,60日で摘果し70日後に各株から果実節葉を採取して分析した6)。メロンの着果枝の基本型は,図6の右に示したように,主茎第13節から伸びた側枝〈子蔓〉の第1節に雌花が着く。近傍の葉をA,B,Cとして左にその形状と色を示す。上段は,着果後40日目に果実を除去した株(30日間は果実無し),下段は最後まで(70日間)果実を残した株,それぞれの葉色である。
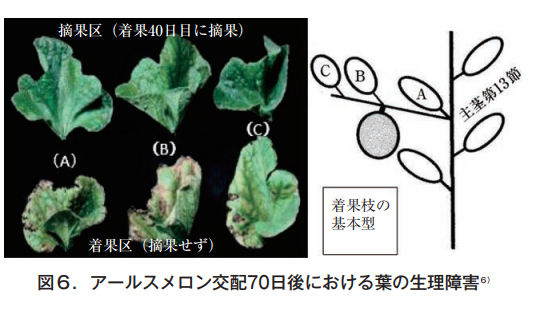
As can be seen from the photo, when the leaves are picked at 40 days, all the leaves remain green, but if not, the leaves are deformed, with chlorosis between the veins and necrosis at the leaf margins. The symptoms are more pronounced in the fruiting node leaves. The fruiting period was set as long as 70 days in order to clarify the relationship between the fruiting period and the presence or absence of fruit. The magnesium, calcium and potassium contents of these leaves are shown in Table 2.
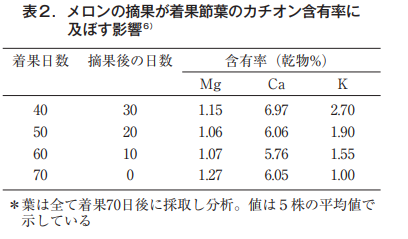
マグネシウムは果実の有無にかかわらず,その値は1.1%~1.2%台でほとんど変化していない。カルシウムは若干低下する傾向にある。これに対して,カリウムは果実が長く存在するほど葉での含有率は低下し,70日間果実を残した株は40日目で除いた株の1/2以下となった。西村ら9)も着果時から経時的に葉分析を行いMg含有率は低下するどころか,むしろ徐々に高まることを明らかにした(図7)。
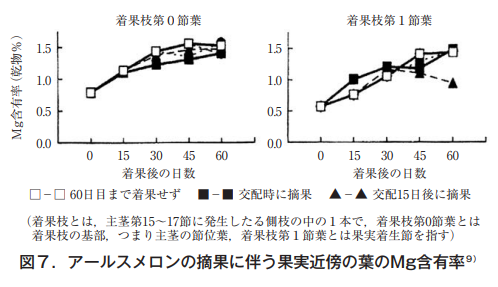
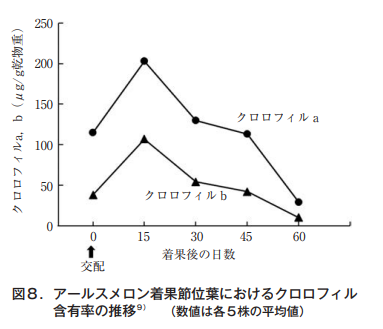
通常,葉は着果40日頃から黄色を帯び始めるが,この変化はクロロフィル値でも知ることができる(図8)。交配15日目に葉色は最も濃くなり,その後,急速に薄れていくことが伺える。
筆者が岡山大学に赴任する前の蔬菜園芸学研究室(益田忠雄教授)では,研究テーマの多くがメロンに設定されていたようである。当時5年間の卒論からマグネシウム,カルシウムおよびカリウムのデータを取り出し成分別に画いてみた。それが図9である。
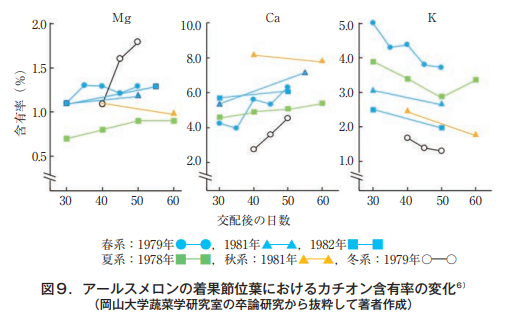
As we will see, magnesium and calcium levels tend to increase gradually with fruit size, while potassium levels clearly decrease. Although intervein chlorosis and leaf margin necrosis would have appeared at 50 to 60 days after crossing, the relationship between the phenomena appearing on leaves and the analytical values has not been discussed. Observation of leaves in the vicinity of fruit set shows that intervein chlorosis caused by Mg deficiency in melons gradually intensifies as the harvest period approaches, so the view that leaf magnesium is transferred to the fruit is reasonable in nature and not at all surprising.
However, there is no evidence to date that magnesium is transferred to fruits. The Encyclopedia of Agricultural Technology in the Rural Electronic Library (National Institute of Agro-Environmental Sciences) also states that magnesium is a mobile component that is transferred from leaves to fruits during fruit enlargement and ripening, and that deficiency symptoms may appear in the leaves around fruits.
Conclusion
Vegetable nutrient physiology, especially nutrient deficiency, is often studied under two conditions: whether or not the same symptoms occur when one component is removed from the complete culture medium, and whether or not the same symptoms occur when other components are increased without removing the component. As shown in Figure 10, it is difficult to attribute leaf deficiency symptoms to a single component because of the interactive effects of ions on nutrient absorption in the roots.
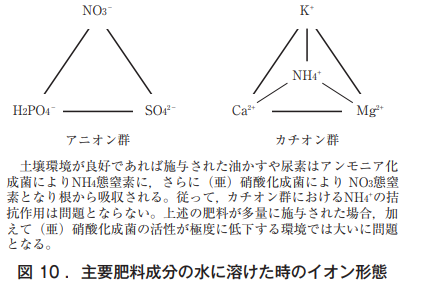
植物の根は,アニオン群,カチオン群から養分を選択的に吸収するが,木部液でのイオン総量〈meq〉は,プラスイオンとマイナスイオンで釣り合っている。土中にはNa⁺,Cl⁻,HCO3⁻などを含め多種の微量要素イオンも存在する。なお,カチオンでは一価が二価よりも吸収されやすいとされ,それらの拮抗作用も知られている(NH4⁺は筆者の栽培経験から挿入)。キュウリの白変葉はカボチャ台木で,かつNH4-N過剰下のMg欠乏により生じ,Mg濃度 が少し高いとグリーンリング葉になりやすい。つまり,白変葉は二つの要素により引き起こされる生理障害であり,一夜にして壊死症状を呈することから,筆者はアンモニアが直接関与している可能性が高いと推察している。
なお,同様な現象はトマトの尻ぐされ症にも認められる。トマトの尻ぐされ症がCa欠乏によって生じることは既知の事実となっているが,NH4-N過剰下のCa欠乏では激しい壊疽(えそ)が果頂部に現れる。2因子以上の要因による生理障害,メロンの着果節の障害葉もクロロフィルの崩壊とカリウムの再移動によって引き起こされる可能性が高いと推察される。
いずれの成分においても果実への再移動に関しては放射性トレーサーを用いてそのエビデンスを得る必要がある。Bukovacら1)は,葉面散布した28Mgは45Caと同様に移動しにくいと記している(ただし,放射性Mgについてはデータ無し)。本稿のキュウリ個葉の散布実験と分析値からマグネシウムは隣の細胞にすら移動しにくいことが確認できた。
最近,田野井11)は,総説「植物のミネラル輸送研究最前線」において,Mg2+トランスポーター遺伝子(MGT)のファミリーがシロイヌナズナ,イネでそれぞれ9個,トウモロコシで12個報告されているとし,それらのマグネシウムの輸送機構について解説しているが,メロンの葉におけるMGT発現量が散布したマグネシウムによって増大しなければ,もしくは,もともと葉におけるMGT機能が微弱であればマグネシウムはその部位に止まり移動しないのも当然と言える。メロン果実近傍の葉の葉脈間クロロシスはクロロフィルの崩壊によって生じているが,葉緑体でフリーとなったマグネシウムが果実に移動しないのもMGT遺伝子の発現量が関係しているかも知れない。
一般に,成熟葉ではマグネシウムの約半分が葉緑体に存在するとされ,その一部が葉緑素構成のマグネシウムであることを考えると,再移動に関してはそのわずかな量を問題にしなければならない。とはいえ,クロロフィル崩壊により生じたフリーのマグネシウムが果実へ再移動するとするには,これまでの実験結果からどう考えても無理がある。28Mgの半減期が約20時間と短いため実験上の制約を受けるにしてもトレーサー法は動態解析に欠かせない手法と言えよう。
Finally, one more question: why does chlorosis occur on the leaves near the fruit as it nears harvest time? If there is no fruit, it would not occur. At this time, some signal must be sent from the fruit to the leaves. What does this signal mean for the melon?
References
1.Bukovac,M.J. and S.H.Wittwer. 1957.
Absorption and mobility of foliar applied nutrients.
Plant Physio. 32:428-435.
2.藤本順子.2008.
園芸作物における栄養障害診断手法の開発と防止対策に関する研究.
島根農技研報. 8:1-45.
3.Jacob,A. 1958.
Magnesium: The fifth majorplant nutrient. 34-54.
Staples Printers Limited,Kingdom.
4.桝田正治.1984.
接ぎ木・自根キュウリの高K培地と高NH4-N培地で発生するMg欠乏症の差異.
農業及園芸59:1051-1053.
5.桝田正治.1989.
キュウリ葉におけるマグネシウムの欠乏症と移動の難易.
岡山大学農学報74:7-14.
6.桝田正治・金沢孝美.1993.
メロン着果節葉のカチオン含有率と養分欠乏症.
園芸学会中四支部大会要旨集32:31p.
7.桝田正治・寺林 敏.2003.
生産技術. 図説野菜新書.矢澤 進(編).97-151.
朝倉書店.東京.〈131pに記載〉.
8.松本満夫・上杉郁夫・柳井利夫:1981.
施設栽培における接ぎ木キュウリのMg欠乏症(グリーンリング症).I.MB剤による硝化抑制が養分吸収とグリーンリング症に及ぼす影響.
高知農林報.13:1-10.
9.西村安代・福元康文・島崎一彦.2004.
アーメロン(Cucumis melo L.)の葉内無機成分に及ぼす着果の影響.
生物環境調節,42:137-146.
10.嶋田典司.1976.
マグネシウムの生理作用.土壌肥料作物栄養大辞典(第3版).102-105.
養賢堂.東京.
11.田野井慶太朗.2021.
マグネシウムの輸送機構.
日本土壌肥料学雑誌.92:108-113.
12.津高寿和.1982.
プリンスメロンの葉枯れ症の原因と対策.
農業及園芸57:1162-1166.
13.山崎 伝.1975.
微量要素と多量要素〈第7版〉.175-183.
博友社.東京.
No Soil - No. 21
「土は生きている」といわれるのはなぜ?
-土は生き物なのか
前 ジェイカムアグリ株式会社
北海道支店 技術顧問
松中 照夫
In this issue, we turn our attention to the soil itself. It is the soil that stably supports agriculture. The soil is the place where food that protects our lives is produced, and at the same time, it nurtures the lives of various living creatures. For this reason, it is often said that "the soil is alive" in awe of it. Why is this so?
1. Is soil a living thing?
When asked again whether soil is really "alive," that is, whether it is a living thing, not many people would say that soil is a living thing.
Living organisms in the general concept are multicellular organisms. To be a multicellular organism, it must satisfy the following three requirements: (1) differentiation and growth, (2) reproduction and heredity, and (3) autonomy in response to environmental changes (Okajima, 1989). However, no one would think that soil has parents who grow up, raise their children, and die by inheriting their genetic elements. Soil is alive" is merely a metaphorical expression of soil. We should be careful not to confuse soil with living beings out of respect for it.
But even if it is a parable, the expression "the soil is alive" does not fail to capture our hearts. Why is that?
2. soil has properties similar to the autonomy of living things
Soil has a property that gives a sense of the autonomy that living things have over their environment, i.e., the ability to maintain their own state of health in the face of external stimuli. This is the buffering power of soil.
Let's take a look at the buffering capacity of soil specifically. Hydrochloric acid and sodium hydroxide are dropped onto the soil as an acidic substance and an alkaline substance, respectively. This means that the soil is stimulated from the outside. As shown in Figure 1, in the case of pure water (H₂O), the pH changes greatly to the acidic side and to the alkaline side with only a small amount of drop.
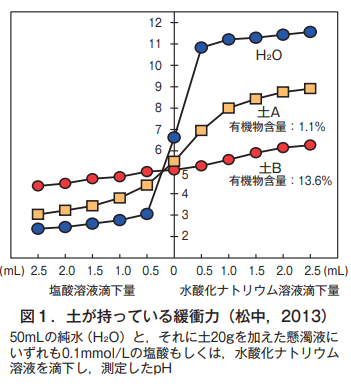
一方,土Aと土Bのどちらも,純水より変化の幅が小さい。つまり,土は純水より緩衝力が大きいのがわかる。ただし,有機物の少ない土Aは,有機物の多い土Bよりも変化の幅が大きく,したがって緩衝力は小さい。土の有機物は,酸性の原因である水素イオン(H⁺)やアルカリ性の原因である水酸イオン(OH⁻)を静電気的に保持する能力を持つ(土の静電気的なイオン保持能については,昨年の2月号の第9回を参照)。このため,有機物がそれらのイオンを保持して動きを抑え,外界からの刺激をやわらげることができる。土Bのほうが土Aよりも緩衝力が大きいのは,土の有機物含量の違いに基づく。このような土が持つ緩衝力は,生き物の自律性とよく似ている。
However, the buffering capacity of the soil is not the only reason why the soil is said to be "alive. The activities of living creatures living in the soil are often invisible to our eyes, and appear to us as if they were performed by the soil itself. This is another major factor that makes us feel that the soil is alive.
3. proof of life of living creatures - soil respiration
The fact that compost fed to the soil becomes nutrients for crops, and that leaves that fall on the soil in the fall and kitchen scraps disappear eventually if they are buried in the soil, are all the result of decomposition of organic matter by soil organisms.
When soil organisms decompose organic matter such as compost, fallen leaves, and garbage, carbon dioxide (CO₂) is released as one of the decomposition products. This is called "soil respiration" because it resembles animal respiration. It is as if the soil is breathing. However, this is only a proof of the activities of living creatures living in the soil, not that the soil itself is breathing.
4. organic matter decomposition is a cooperative play among soil organisms
Using the fallen leaves mentioned above as an example, let us take a look at the beautiful interplay between soil organisms as they decompose organic matter (Fig. 2).
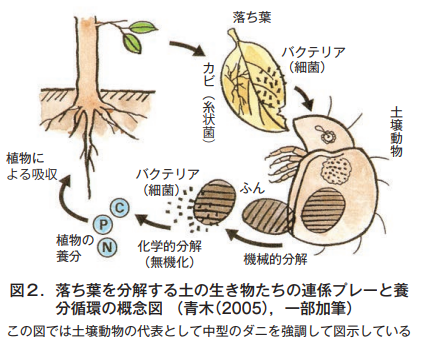
Fallen leaves (plant remains) on the soil surface do not change significantly when they remain dry. However, once they are wetted by rain, bacteria (bacteria) and fungi (filamentous fungi) attach to them and soften the plant remains to some extent.
Then, large soil animals such as earthworms, borers, and sowbugs appear and feed on the plant remains, pulverizing them and dragging them and their remains into the soil. Medium-sized soil animals, such as mites and stoneflies, then take charge, feeding on the dragged organic matter and taking nutrients into their bodies, and excreting the unwanted material as feces.
Bacteria and fungi feed on the excreted feces and the remains of plants that have been dragged into the soil, eventually transforming them into carbon dioxide, water, and inorganic substances. The inorganic matter produced by this process is absorbed by the roots as nutrients for the plants growing there. Thus, a cycle of nutrients is established. Of course, the same mechanism applies to animal remains as it does to plants.
The various material changes that occur in the soil include changes involving soil organisms. Therefore, when we talk about material changes in the soil, if we blur the distinction between the function of the soil and the function of living creatures in the soil, we confuse the two.
5. distinguish between the workings of the soil and the workings of living things.
The expression "the action of the soil" does not mean that the soil has a "will (purposiveness)" like a living creature and that the soil itself actively works by its own will. The expression "the soil supplies nutrients to the crop" is also inappropriate, because the soil itself does not intentionally supply nutrients to the crop. When the crop selects and absorbs the nutrients it needs, such as cations, from among the various nutrient ions dissolved in the water (soil solution) in the soil, the balance of the electro-neutral principle (equal charge of cations and anions) is upset. Various reactions take place in the soil to restore the imbalance, and cations are released into the soil solution to maintain the electroneutrality principle.
This is the phenomenon described as "the soil supplying nutrients to the crop. This reaction is also governed by a certain principle (in this case, the principle of electro-neutrality in solution), and there is no room for the free will of the creature.
I would like to understand the true meaning of the parable "the soil is alive" after distinguishing the function of the soil and the creatures in the soil.