

Site Search
Search within product
No. 754 Published 2023 (R05) .10
Click here for PDF version
農業と科学 令和5年10月
本号の内容
§ In Ibaraki Prefecture's lotus root cultivation
窒素適正施肥技術の開発
Horticulture Research Institute, Ibaraki Prefectural Agricultural Research Center
Soil Fertilizer Laboratory
鹿島 啓司
§酸性デタージェント可溶有機態窒素含量を用いた
有機質資材窒素肥効見える化の取り組み
Kyushu-Okinawa Agricultural Research Center, National Agricultural Research Organization
Warm-Stand Livestock Research Area
主席研究員 古賀 伸久
§土のはなし-第25回農業が環境破壊の始まり
-人間活動と環境との関わり-
前 ジェイカムアグリ株式会社
北海道支店 技術顧問
松中 照夫
Ibaraki Prefecture in the cultivation of lotus root
窒素適正施肥技術の開発
Horticulture Research Institute, Ibaraki Prefectural Agricultural Research Center
Soil Fertilizer Laboratory
鹿島 啓司
Introduction
The area planted with lotus root in Ibaraki Prefecture is 1,710 ha (Kanto Agricultural Administration Bureau, Ministry of Agriculture, Forestry and Fisheries, 2021), the largest in Japan. The main production areas in the prefecture are adjacent to the Kasumigaura and Kitaura lakes and marshes, and there is concern that fertilizer runoff from lotus root fields may affect the water quality of these marshes. In addition, fertilizer prices have risen sharply in recent years, increasing the need for fertilizer reduction technology.
Therefore, in this prefecture, we have worked on the development of nitrogen-optimized fertilization techniques for lotus rootstocks, and have developed three effective techniques. The results were compiled into a "Manual for Proper Fertilizer Application to Lotus japonicus (Fig. 1; Agricultural Technology Division, Ibaraki Prefecture, 2022).

2. development of nitrogen-optimized fertilization technology
(1) Development of fertilizer with regulated fertilizer efficacy according to its absorption characteristics
Until now, there has been little knowledge on the growth process and nutrient absorption of lotus rootstocks, with only a series of reports by Komatsu et al. (1980, 1981) on a late maturing branch variety. The fertilizer system for lotus rootstocks in this prefecture was constructed based on these findings. On the other hand, the main varieties grown in Ibaraki Prefecture today are early maturing varieties, and the cultivation environment, such as weather conditions, is different from that of those days. Therefore, from 2012 to 2014, we investigated nutrient absorption during growth to clarify the fertilizer absorption characteristics of early-maturing varieties of lotus rootstocks, which are currently the mainstream. The results revealed that nitrogen absorption was low until mid-June, the early stage of growth, and increased significantly toward the end of August (Odabe and Iimura, 2015).
Based on these results, we collaborated with a fertilizer manufacturer to develop a coated urea-based fertilizer with reduced initial nitrogen leaching. The nitrogen leaching rate of the fertilizer was designed to match the nitrogen absorption characteristics of lotus rootstocks, and to slightly exceed the nitrogen absorption rate at any time during the growing period (Figure 2). This fertilizer with regulated fertilizer effect is thought to make it possible to supply fertilizer according to the nitrogen absorption pattern of lotus rootstocks and to apply fertilizer with less waste (Odabe and Iimura, 2018).
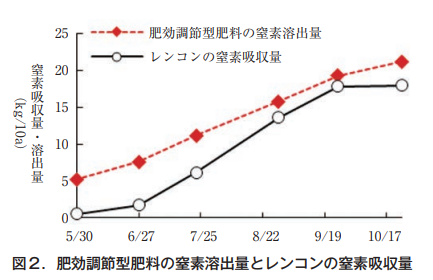
(2) Elucidation of the fertilizing effect of lime nitrogen and development of specialized fertilizers
In lotus root cultivation, lime nitrogen is sometimes applied before planting to control pests. This lime nitrogen is both a pesticide and a fertilizer containing 20% nitrogen. However, the fertilizing effect of lime nitrogen in lotus root cultivation has not been clarified, and in the field, fertilizers have been applied without considering this effect. Therefore, from FY 2015 to FY 2017, cultivation trials using lime nitrogen were conducted to clarify the nitrogen fertilization effect of lime nitrogen in lotus root cultivation.
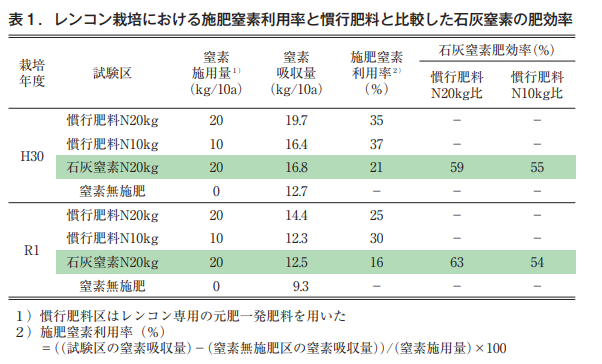
As a result of growing lotus rootstocks using conventional fertilizer and lime nitrogen as nitrogen fertilizer and investigating nitrogen absorption, it was found that the nitrogen in lime nitrogen had about 50% higher fertilizer efficacy than that in conventional fertilizer (Table 1; Kariya et al., 2023). In addition, its fertilizing effect reaches its maximum 40 to 50 days after the application of lime nitrogen and is maintained from June to September, when the nutrient absorption of lotus rootstocks is vigorous (Ibaraki Prefectural Agricultural Research Center, Horticulture Research Institute, 2017).
The application of lime nitrogen at 100 kg/10 a has been shown to reduce the density of the common lentil pest, Lentinus edodes (Ibaraki Prefectural Agricultural Research Center, Horticulture Research Institute, 2016). Therefore, we applied lime nitrogen under these conditions in the local field, and cultivated a reduced-fertilizer zone where the fertilizer effect of lime nitrogen was reduced from that of the conventional fertilizer application.
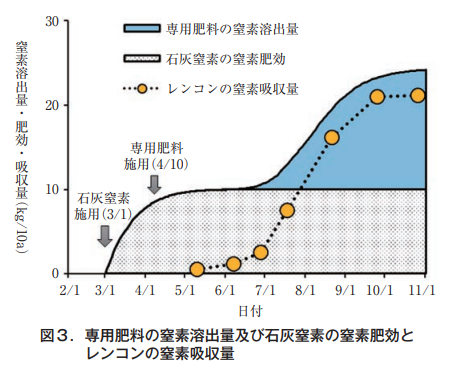
Based on these results, we developed, in cooperation with a fertilizer manufacturer, a fertilizer with a regulated fertilizer effect (hereinafter referred to as "special fertilizer") based on the application of lime nitrogen. The nitrogen contained in the special fertilizer is a slow-release type that suppresses initial leaching. By applying 100 kg/10a of lime nitrogen and 160 kg/10a of the special fertilizer, the combined amount of the fertilizing effect of the lime nitrogen and the nitrogen leaching amount of the special fertilizer slightly exceeds the amount of nitrogen absorbed by lotus root crops at any time during the growing period (Figure 3), thus allowing fertilizer application without excess or deficiency. In addition, the price is relatively inexpensive, and fertilizer costs can be reduced (Ibaraki Prefectural Agricultural Research Center, Horticulture Research Institute, 2019).
(3) Development of diagnostic fertilization technology and evaluation of its practicality
Inorganic nitrogen in the soil exists in the ammonia form because most lotus root fields in this prefecture are flooded with water throughout the year. The amount of ammonia nitrogen remaining in the soil of lotus root fields varies greatly from field to field.
different (Figure 4).
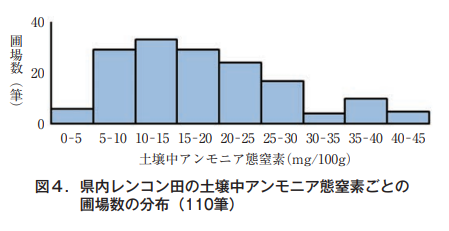
The authors focused on ammonia nitrogen in the soil of lotus root fields, and also considered the fertilizing effect of lime nitrogen as mentioned above.
We have developed a diagnostic fertilization technique that takes into account the soil fertility and the amount of lime nitrogen applied. The diagnostic fertilization formula shown in Figure 5 allows growers to calculate the optimal amount of nitrogen to be applied to the growth of lotus root crops, depending on soil fertility and the amount of lime nitrogen applied.

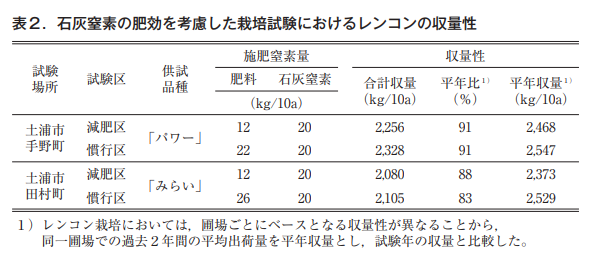
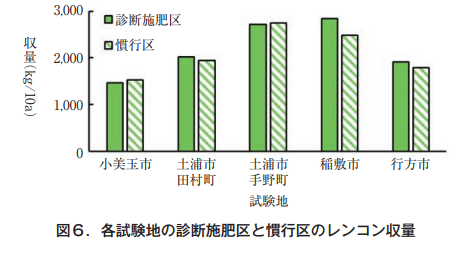
Cultivation tests were conducted in a local lotus root field with a diagnostic fertilizer application area in which the amount of nitrogen applied was determined by a diagnostic fertilizer application formula and a conventional area. The amount of nitrogen applied in the diagnostic fertilizer application areas ranged from 5 to 20 kg/10a, which was 11 to 78% less than the 23 to 24 kg/10a of nitrogen applied in the conventional areas. In each test site, the yield of the diagnostic fertilization area was demonstrated to be equal to or higher than that of the conventional area (Fig. 6: Horticulture Research Institute, Ibaraki Prefectural Agricultural Research Center, 2022).
3. efforts toward practical application of nitrogen-optimized fertilization technology
(1) Development of soil sampling methods for diagnostic fertilizer application technology
In order to utilize diagnostic fertilization techniques, it is necessary to measure ammonia nitrogen in soil, and we developed a simple soil sampling method using a familiar instrument. Using a general transplanting trowel (tip length of about 17 cm), we collected uniformly up to 30 cm of soil by the following method. First, the surface layer (0 to 15 cm) of the soil is sampled, and then the lower layer (15 to 30 cm) is sampled in approximately the same amount.
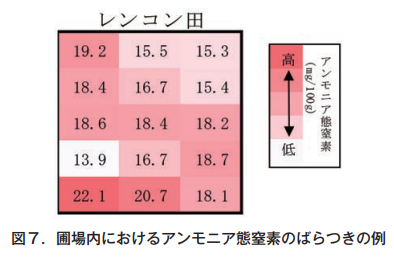
Ammonia nitrogen in soil can vary greatly from one sampling point to another, even within a single plot (Figure 7). To accurately evaluate ammonia nitrogen in a field, it is important to collect the same amount of soil from each of the four corners and five points in the center of the field, mix them well, and use them as soil samples for that field (Horticulture Research Institute, Ibaraki Prefectural Agricultural Research Center, 2022).
(2) Development of a simple evaluation method for ammonia nitrogen in soil that can be implemented at extension centers
Guidance agencies such as extension centers are sometimes required to quickly measure ammonia nitrogen, but the equipment needed to measure ammonia nitrogen is expensive and often not owned by the agencies. Therefore, we developed a simple evaluation method that does not require expensive equipment.
In the specific evaluation procedure (Fig. 8), a provisional specific gravity estimate is obtained from the soil volume after adding a 10% potassium chloride solution to air-dried soil, shaking the solution, and allowing it to stand still. The extracted solution is then colored using a simple water quality measurement kit and measured with a simple absorption spectrophotometer to obtain an estimate of ammonia nitrogen per air-dried soil. The ammonia nitrogen per area can be estimated from the provisional specific gravity obtained in this way and the estimated ammonia nitrogen per air-dried soil (Kashima et al., 2023).

(3) Analysis of ammonia nitrogen in soil in cooperation with JA Zen-Noh Ibaraki
It is difficult for extension centers and other guidance institutions to handle soil analysis for the entire production area. Therefore, in cooperation with JA Zen-Noh Ibaraki, which is responsible for soil analysis in many production areas, we added ammonia nitrogen as one of the analysis items for lotus root field soil.
The prescription presented after soil analysis will include the appropriate amount of fertilizer calculated based on the diagnostic fertilizer application technique.
By using this prescription as a guide, growers can reduce fertilizer application costs while maintaining yields.
Summary
In recent years, the Sustainable Development Goals (SDGs) have been attracting attention. In lotus root cultivation, the three nitrogen-optimized fertilization techniques introduced here can be used to reduce fertilizer application costs and stabilize production while reducing the burden on the environment. By continuing to provide guidance, we hope to contribute to the sustainable production of lotus rootstocks and the development of production areas in this prefecture.
5. cited references
●茨城県農業技術課(2022)れんこん適正施肥マニュアル.
●茨城県農業総合センター園芸研究所(2016)
レンコンネモグリセンチュウに対する総合防除法.
Research Results of the Horticulture Research Institute, Ibaraki Prefectural Agricultural Research Center
●茨城県農業総合センター園芸研究所(2017)
レンコン栽培における石灰窒素の窒素肥効特性.
Research Results of the Horticulture Research Institute, Ibaraki Prefectural Agricultural Research Center
●茨城県農業総合センター園芸研究所(2022)
レンコン田土壌のアンモニア態窒素を評価するための簡易な土壌採取法.
Research Results of the Horticulture Research Institute, Ibaraki Prefectural Agricultural Research Center
●茨城県農業総合センター園芸研究所(2022)
レンコン田土壌のアンモニア態窒素を考慮した窒素適正施肥法.
Research Results of the Horticulture Research Institute, Ibaraki Prefectural Agricultural Research Center
●茨城県農業総合センター園芸研究所・農業研究所(2019)
レンコンにおける石灰窒素の肥効および
養分吸収特性を考慮した窒素施肥法.
Research Results of the Horticulture Research Institute, Ibaraki Prefectural Agricultural Research Center
●假屋哲朗・藤田 裕・小田部 裕・寺門 巌・飯村強(2023)
レンコン栽培における石灰窒素のアンモニア化特性と窒素肥効の推定.
茨城県農業総合センター研究報告5:1-9
●鹿島啓司・假屋哲朗・寺門 巌・郷内 武・藤田裕(2023)
レンコン田土壌のアンモニア態窒素の簡易評価法.
茨城県農業総合センター研究報告5:45-51
●小松鋭太郎・篠崎佳信・石塚由之(1980)
種バスの萌芽伸長に関する幾つかの条件について.
茨城県園芸試験場研究報告8:19-32
●小松鋭太郎・篠崎佳信・石塚由之(1981)
食用ハスの生育経過と養分吸収.
茨城県園芸試験場研究報告9:7-22
●農林水産省関東農政局(2021)作況調査(野菜)
●小田部 裕・飯村 強(2015)
茨城県におけるレンコン主要品種の乾物生産および養分吸収特性.
日本土壌肥料学雑誌86(4):283-289
●小田部 裕・飯村 強(2018)
収量および養分吸収特性に応じたレンコンの合理的施肥法.
日本土壌肥料学雑誌89(3):220-226
Using acid detergent soluble organic nitrogen content
有機質資材窒素肥効見える化の取り組み
Kyushu-Okinawa Agricultural Research Center, National Agricultural Research Organization
Warm-Stand Livestock Research Area
主席研究員 古賀 伸久
Introduction
Along with chemical fertilizers, organic materials such as livestock manure, vegetable oil cake, and rice bran are sources of fertilizer components (nitrogen, phosphate, potash, etc.) for crops. The recent sharp rise in fertilizer prices has drawn attention to the fertilizer components of organic materials. In May 2021, the Ministry of Agriculture, Forestry and Fisheries (MAFF) formulated the "Green Food System Strategy," which aims to realize both improvement of productivity and sustainability of food, agriculture, forestry, and fisheries industries through innovation. (Law Concerning the Promotion of Environmentally Friendly Business Activities to Establish a Food System in Harmony with the Environment) was enacted in July 2022.
The strategy sets the following goals for the agricultural sector by 2050: (1) to achieve zero CO₂ emissions in agriculture, forestry, and fisheries; (2) to reduce the use of chemical pesticides by 50%; (3) to reduce the use of chemical fertilizers by 30%; and (4) to increase the ratio of the area devoted to organic agriculture to 25%. With regard to goals (3) and (4), organic materials will play an important role as a substitute for chemical fertilizers.
In chemical fertilizer reduction and organic cultivation, the application of organic materials plays an important role in terms of nutrient supply, but most of the nitrogen in organic materials is in the organic form1) , which is an important element for crops, and crops cannot absorb and utilize nitrogen in its original form. The organic nitrogen in organic materials is applied to the soil and converted to inorganic nitrogen (ammonia-form nitrogen and nitrate-form nitrogen) by the action of microorganisms, and only then can it be used by the crop plants. The phenomenon of releasing inorganic nitrogen through the decomposition of organic nitrogen in organic materials is called nitrogen mineralization, which involves several factors, including soil environmental factors such as soil temperature and soil moisture, and the characteristics of the organic material itself. Some organic materials contain large amounts of phosphate and potassium, and the application of organic materials can reduce the amount of phosphate and potassium fertilizers.
Recently, the acid detergent soluble organic nitrogen (ADSON) content has been attracting attention as a characteristic value of organic materials that is highly related to nitrogen mineralization of organic materials. It was found that ADSON content in organic materials has a high positive correlation with the amount of inorganic nitrogen produced as a result of decomposition of organic materials, and examples of research applying this finding to the evaluation of nitrogen fertilizer efficacy of organic materials have been reported4),5) . ADSON, an organic nitrogen component dissolved in acid detergent solution (sulfuric acid solution with surfactant), is considered to represent an organic nitrogen component that is relatively easy to decompose. Therefore, we constructed a prediction model for nitrogen mineralization of organic materials using ADSON content of organic materials as an input variable in addition to soil temperature and soil moisture, and developed a web application to visualize the nitrogen fertilizer effect of organic materials so that anyone can know the nitrogen fertilizer effect of organic materials with a simple input process.
2. characteristics of ADSON content for each organic material
As mentioned earlier, it has already been reported that ADSON content has a high positive correlation with the amount of inorganic nitrogen produced by the decomposition of organic materials4),5) . Therefore, we collected about 500 organic materials, including livestock manure (cattle manure, swine manure, and chicken manure), commercial organic materials (rape oil cake, fish meal, rice bran, etc.), green manure, and crop residues, mainly in the Kyushu-Okinawa area, and examined their ADSON content (Figure 1).
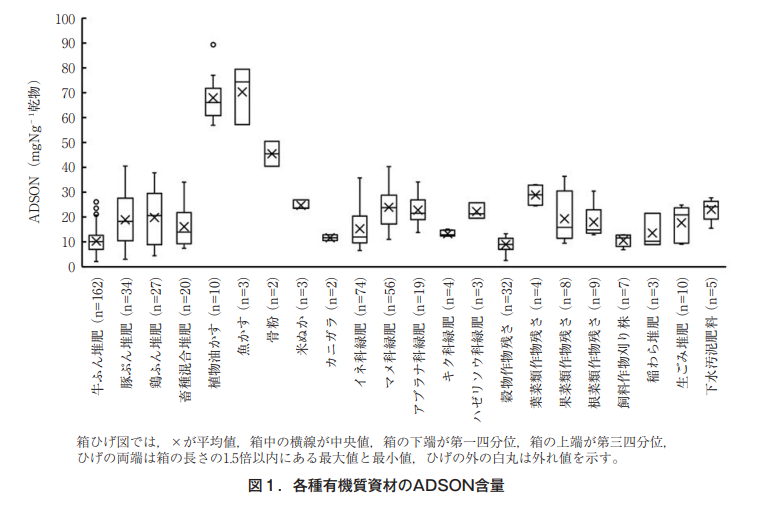
The ADSON content (unit: mgNg-1 dry matter) of organic materials was higher in vegetable oil cake such as rapeseed oil cake and soybean oil cake, and fish meal. Among livestock manure, ADSON content was similar in swine manure and chicken manure. The median value was about 20 mg Ng-1 dry matter, but there was a large variation. For the swine manure, the ADSON content was similar for the two main raw material ratios
(It has been shown that the ADSON content increases as the ratio of composting material (livestock manure is composed of manure as the main material and secondary materials such as sawdust, and the ratio of main material indicates the weight percentage of manure in the compost) increases1) . The median ADSON content of cattle manure compost was about 10 mg Ng-1 dry matter, but according to Koyanagi et al.5) , when ADSON content is less than 10 mg Ng-1 dry matter, nitrogen mineralization of organic materials is reported to be zero or negative (organicization). Therefore, it is expected that about half of the cattle manure would not mineralize nitrogen.
Among green manures, legumes (e.g., hairy vetch, crotalaria, crimson clover) and cruciferous green manures (e.g., white mustard) are slightly higher, while grasses (e.g., tritical whole plants, oat, sorghum, sorgha) are lower.
The ADSON content of crop residues (e.g., wheat and soybean) is slightly lower. The ADSON content of crop residues was highest in leafy vegetable crop residues (stover of broccoli, cabbage, etc.) and lowest in grain crop residues (stover of wheat, soybean, buckwheat, seed corn, etc.) and stubble of forage crops (Sudan grass, etc.). The ADSON content of food waste compost and sewage sludge fertilizer was similar to that of swine manure and chicken manure.
3. construction of a model for nitrogen mineralization of organic materials by introducing ADSON content
Soil environmental factors such as soil temperature and soil moisture, and material characteristics such as ADSON content and C/N ratio (carbon content) are involved in the mineralization of nitrogen in the soil. We attempted to construct a statistical model to predict the amount of inorganic nitrogen produced in the soil if the soil temperature, soil moisture, and ADSON content of the organic material applied during the period from material application to harvest were known. Thirty-two organic materials were incubated for 1, 4, and 12 weeks under different soil conditions of temperature (10, 20, and 30°C) and moisture (45, 60, and 75% of maximum water capacity), and the amount of inorganic nitrogen produced at that time was determined and used as observed data.
The observed data were treated as the first-order reaction equation of a simple model, and the amount of inorganic nitrogen produced from organic materials was assumed to increase exponentially with the passage of incubation time. By applying hierarchical Bayesian estimation to the observed data, parameters such as decomposition rate constants were estimated, and this was used as a statistical model (organic material nitrogen mineralization model)2) . The model showed that the maximum amount of nitrogen mineralization was greater for materials with higher ADSON content (Fig. 2).
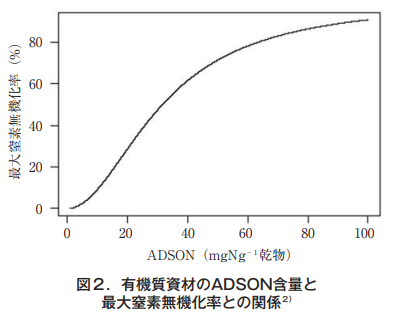
It was also confirmed that the temperature response was small for materials with ADSON content of 30 mgNg-1 dry matter or less, and that the effect of soil moisture on nitrogen mineralization was small2). In the validation of the model in open fields, it was confirmed that the model underestimates the estimated values for about one month after the application of organic materials3), and we will work to improve the model in the future.
4. release of an application for visualization of nitrogen fertilizer efficacy of organic materials
The organic material nitrogen mineralization model itself is difficult for the general public to use because it operates using a programming language. Therefore, we have developed an application of the organic material nitrogen mineralization model and made it available on the web (Fig. 3), so that anyone can obtain the nitrogen fertilization effect of organic materials with a simple input process. This app is available on the Japan Soil Inventory Soil Management Apps Collection website (https://soil-inventory.rad.naro.go.jp/main/organic-fertilizer). The app can be operated with a PC or a smartphone (Fig. 4).
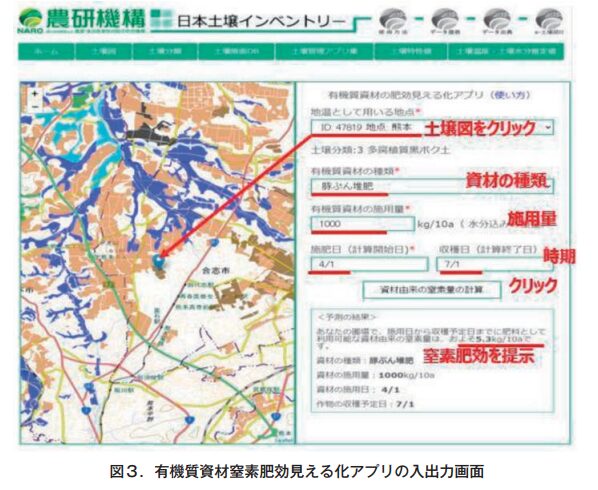

The left half of the input screen is a digital soil map, and the farmland to which organic materials are to be applied is selected from the map. By selecting a farmland on the soil map, daily soil temperature and soil moisture are retrieved, which are calculated in advance from the nearest AMeDAS weather data (temperature and precipitation data for the past 30 years). Next, the type of material is selected. The user can select from 14 types of materials, including cattle manure, swine manure, chicken manure, vegetable oil cake, fish meal, rice bran, and green manure. By selecting a material, the model parameters and material property values required for the calculation (the average values from a multi-point survey of moisture content and ADSON content are used as default values) are automatically called.
Then, the amount of organic material applied per 10a, the date of material application (calculation start date), and the harvest date (calculation end date) are entered. After pressing the Calculate button, the amount of inorganic nitrogen per 10a produced from the date of material application to the harvest date (i.e., the amount of nitrogen available for fertilizer reduction) is output. In organic cultivation, where all the nutrients required by the crop are supplied from organic materials, this application can be used to determine the amount of inorganic nitrogen supplied from organic materials, which can be used as a reference when determining the amount of materials to be applied. In the case of reduced chemical fertilizer cultivation, the amount of inorganic nitrogen supplied from organic materials can be used to determine how much chemical fertilizer should be used to compensate for nutrient deficiencies.
It should be noted that this application is limited to use in fields that are not flooded, and an application that can be used in flooded rice paddies is currently under development (as of September 2023). It should also be noted that default values are used for water content and ADSON content. Even for cattle manure, water content and ADSON content can vary widely depending on the production method, type of secondary materials, composting period, and other factors1). 1) There is a difference between the characteristic values of organic materials actually applied to farmland and those prepared as default values, and it is important to understand that this difference may cause errors in the prediction results when using this application.
5. at the end
It is not easy to supply exactly the nutrients needed by crops with organic materials, because nitrogen mineralization of organic materials is itself strongly affected by environmental conditions such as soil temperature and material characteristic values. However, this application makes it possible to visualize the effect of nitrogen fertilizer. Even in organic and chemical fertilizer-reduced cultivation, excessive application of organic materials increases environmental burdens such as eutrophication of soil, nitrate contamination of groundwater, and generation of dinitrogen monoxide, a greenhouse gas.
On the other hand, it is not hard to imagine that insufficient application of organic materials would result in lower yields. The combined use of chemical fertilizers and organic materials is a realistic agricultural method that conserves resources and improves the sustainability of agriculture. We hope that this application will contribute to the realization of the vision of the "Green Food System Strategy" by making fertilizer management smarter and more environmentally friendly by using organic materials as fertilizers.
References
1.古賀伸久・新美 洋・井原啓貴・山口典子・山根 剛・草場 敬2019.
各種有機質資材における酸性デタージェント可溶有機態窒素含量
-資材毎の特徴およびC/N比との関係-.土肥誌,90,107-115.
2.古賀伸久・仁科一哉・望月賢太・小林創平・
新美 洋・井原啓貴・山口典子・山根 剛・草場 敬2023.
酸性デタージェント可溶有機態窒素含量を導入した
有機質資材窒素無機化予測モデルの構築.土肥誌,94,106-114.
3.望月賢太・小林創平・井原啓貴・渕山律子・古賀伸久・仁科一哉2023.
畑条件における有機質資材窒素無機化予測モデルの検証.
土肥誌,94,179-186.
4.小柳 渉2017.
AD可溶窒素とアンモニア態窒素を指標とした有機質資材の窒素肥効評価
第2報 適用性の拡大と速効性窒素の推定について.
土肥要旨集,63,132.
5.小柳 渉・平尾賢一・棚橋寿彦2016.
AD可溶窒素とアンモニア態窒素を指標とした有機質資材の窒素肥効評価.
土肥要旨集,62,121.
No Soil - No. 25 Agriculture is the Beginning of Environmental Destruction
-人間活動と環境との関わり-
前 ジェイカムアグリ株式会社
北海道支店 技術顧問
松中 照夫
Soil is created in a given environment through the work of the living organisms present there. This is the idea of the Russian soil scientist Dokuchaev, which I mentioned last month. As if to prove the correctness of this idea, from a macroscopic viewpoint, the world's soil is distributed along latitudinal zones corresponding to climatic changes. This is because climatic conditions have a great influence on the environment. We can truly feel that soil is a product of the environment.
This month, we will consider the relationship between the soil and the environment in terms of human activities, especially in terms of food production.
1. the beginning of agriculture
It was the Paleolithic Cro-Magnon man who, about 40,000 years ago, tried to secure their own food by using the soil (Tadge, 2002). They obtained their food in a different way from the Neanderthals, who obtained their food by hunting and gathering. They did not hunt animals haphazardly, but rather, based on the habits of the animals, they thought about where to ambush them. They created a new way of hunting, in which they divided the roles among themselves, tracked a herd of animals, surrounded it, and captured it. They also created new and varied tools. At the same time, they protected and cultivated plants for their food. This was a precursor to the beginning of agriculture. It is believed that about 10,000 years ago, life began to incorporate what we now call agriculture (Ponting, 1994).
This beginning of agriculture was an active act of man working with nature to clear the land and grow food in its soil. It changed the way people lived. Of course, at that time, the natural environment must have had an overwhelming power over mankind. However, it was the first time that mankind challenged nature. In the sense that humans added their hands to the given natural environment, it can be understood as the beginning of environmental destruction (Figure 1). At a stage when human activities were weaker than the forces of the natural environment, this was not a major problem.

2. the center of human productive activity changed with the Industrial Revolution
However, when human activities become so active that they alter the natural environment, the altered natural environment cannot be restored. It was the Industrial Revolution that reversed the power relationship between human productive activities and nature.
From the late 18th century to the first half of the 19th century, the Industrial Revolution in England transformed the way people lived.
People at that time, who learned to mechanize energy conversion using coal, took advantage of it to greatly develop their economic activities. Until then, agriculture had been the center of human productive activity. However, the Industrial Revolution converted it into industry.
Around the same time as the Industrial Revolution, the hyper-intensive Norfolk farming system was established. This led to a dramatic increase in agricultural productivity. This was a factor in increasing the rural population. Ironically, the increased rural population was sent to the cities as factory workers, which were needed in the Industrial Revolution. This led to a rapid increase in the urban population. The total population of pre-industrial England in 1801 was 8.32 million. The urban population was 3.66 million, or 44% of the total population. Eighty years later, in 1881, after the Industrial Revolution, the total population had tripled to 25.91 million, and the urban population had quintupled to 17.28 million, 67% of the total population.
3. commercialization of food has changed the value of food
Both to increase the population and to provide food for the increased population, it is necessary to increase the production of food. The increase in the production of food was demanded in the rural areas. This situation further led to the enclosure of farmland. At the same time, farm management, in which capitalists who leased large tracts of land from landlords hired peasants as wage laborers to turn the food they produced into a commodity and make a profit from its sale, emerged and expanded. Thus, with the Industrial Revolution, communal subsistence management disappeared from English farming villages. This transformation of rural society that occurred with the Industrial Revolution in England in the latter half of the 18th century is called the Agricultural Revolution.
This change has profoundly altered the nature of the economy since then. Until then, in subsistence farming operations, the important factor was that food was useful to people by satisfying their hunger (this was called use value). On farms run by capitalists, however, the objective is to produce food as a commodity for the wage earners who are the consumers. For this reason, how much of the food produced is sold for and how much profit is earned (this is called the exchange value) becomes important. In other words, the value of the food produced shifted from use value to exchange value.
The costs that agriculture had incurred in the past to maintain productivity by conserving resources common to all humankind, such as air, water, and soil, became negative costs in the short term in terms of increased profits. The idea that these costs could be passed on to the natural environment and that agriculture, which consumes resources free of charge, would be more profitable, gradually gained ground.
4. criticizing plundering agriculture without nutrient cycle, Liebig
It was Liebig, who lived during the Industrial Revolution, who strongly criticized this waste of resources, especially agriculture, which only takes crop nutrients from the soil and does not replenish them, thereby reducing the fertility of the soil.
After the Industrial Revolution, the division of labor between rural and urban areas progressed in England. Grain, the staple food of the countryside, was produced in rural areas, and the grain was consumed by urban workers in London and other cities. Crop nutrients absorbed from the soil of rural farmland were transported to the cities in the form of harvested grains. Some of the crop nutrients in the grain eaten by city workers were transferred to the workers' excrement, which was thrown out of their windows onto the streets at night. Since cities at that time did not have sewerage systems, they were carried away by the rain and, in London, directly into the Thames.
As a result, the Thames was filled with a strong stench and was extremely unsanitary. Thus, the crop nutrients in the rural soil could not return to the soil of the original farmland. Liebig strongly criticized "plunder agriculture," which only consumed the nutrients of the soil, as being unable to provide a stable food supply, and emphasized that the most important factor for agricultural sustainability was the nutrient cycle.
Liebig's ideal of nutrient cycling was Japanese agriculture. In the Edo period (1603-1867), the pathways by which the urine of consumers in the then world-class metropolis of Edo was returned to the farmlands of the surrounding farming villages that provided them with food were well established from the 17th century onward (Liebig and Yoshida, 2007).
Since the Industrial Revolution, people's lives have become more affluent and convenient, and human activities have increased (Figure 2). The soil, which is supposed to be the common property of society entrusted to us by nature, has given way to selfish economic activities in which resources are used and disposed of as they see fit. Nowadays, such activities have even led to the problem that agriculture itself has become a source of environmental pollution. We will look at the specific problems in next month's issue and thereafter.
